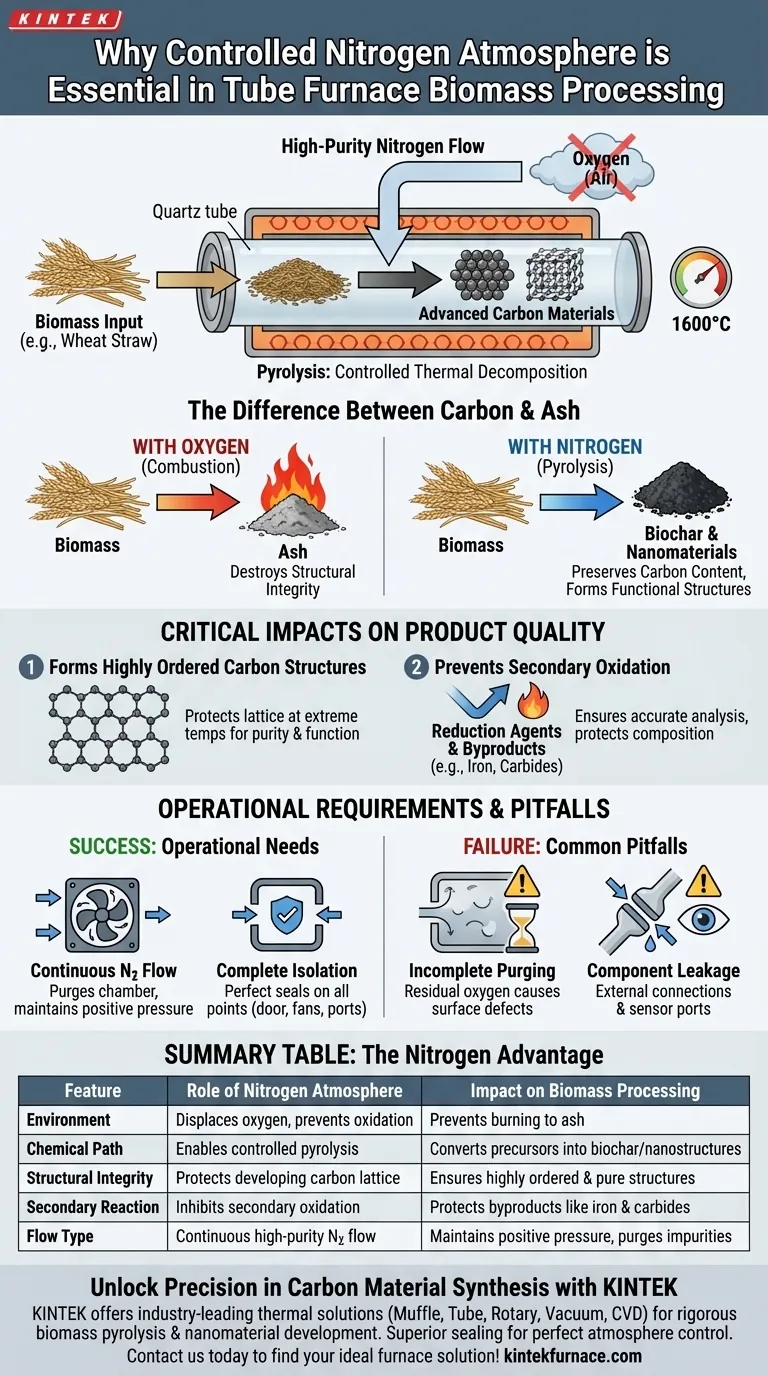
A controlled nitrogen atmosphere is the defining factor between creating advanced carbon materials and simply burning biomass to ash. In a tube furnace, this inert environment displaces oxygen to prevent combustion, ensuring that high-temperature processing converts precursors (like wheat straw) into pure, highly ordered carbon structures rather than destroying them.
Core Insight: The presence of oxygen during high-heat treatment triggers combustion, destroying the material's structural integrity. Nitrogen acts as a protective barrier, forcing the biomass to undergo pyrolysis—a thermal decomposition process that preserves carbon content and creates functional nanomaterials.

The Chemistry of Inert Processing
Preventing Oxidation and Combustion
The most immediate function of nitrogen is to create an oxygen-deficient environment.
If biomass is exposed to high temperatures (up to 1600°C) in the presence of air, it will ignite and oxidize.
Nitrogen displaces room air, preventing this direct combustion and ensuring the biomass survives the thermal shock.
Enabling Selective Thermal Degradation
Instead of burning, the biomass undergoes controlled degradation.
The inert atmosphere facilitates specific chemical changes, such as dehydration and decarboxylation.
This selectively breaks down cellulose, hemicellulose, and lignin, leaving behind a carbon-rich solid known as biochar.
Critical Impacts on Product Quality
Forming Highly Ordered Carbon Structures
For advanced applications, such as converting wheat straw into nano carbon spheres, purity is paramount.
The nitrogen atmosphere protects the developing carbon lattice as it forms at extreme temperatures.
This ensures the final product retains specific functional properties and high structural order, which would be ruined by oxidation.
Preventing Secondary Oxidation
The protective role of nitrogen extends beyond the biomass itself.
It prevents the unintended combustion of carbon reducing agents and protects newly formed byproducts, such as metallic iron and chromium carbides.
Without this barrier, these materials would undergo secondary oxidation, altering their chemical composition and rendering analysis inaccurate.
Operational Requirements for Effectiveness
Establishing a Continuous Flow
A static volume of nitrogen is rarely sufficient.
An atmosphere control system must provide a continuous flow of high-purity nitrogen to purge the chamber and maintain a positive pressure barrier against the outside environment.
Ensuring Complete Isolation
The effectiveness of the atmosphere relies entirely on the furnace's seal.
The internal working space must be isolated using sealing devices on the shell, door, and all connection points (fans, thermocouples, pushers).
Even a minor leak can introduce enough oxygen to degrade the sample quality.
Common Pitfalls to Avoid
Incomplete Purging
Failing to fully displace the initial volume of air before heating begins is a critical error.
Residual oxygen trapped in the tube will attack the biomass surface immediately as temperatures rise, leading to surface defects or partial ash formation.
Ignoring Component Leakage
Users often focus on the main door seal but neglect peripheral connections.
External connecting parts like radiation tubes and sensor ports are common failure points where air can be inhaled, compromising the inert environment.
Optimizing Your Thermal Process
To achieve the desired material properties, align your atmospheric control with your specific output goals:
- If your primary focus is High-Purity Nanomaterials: Ensure a continuous, high-volume nitrogen flow to support processing up to 1600°C without structural degradation.
- If your primary focus is Chemical Analysis: Prioritize the isolation of the furnace chamber to prevent secondary oxidation that could skew your data on reduction products.
Ultimately, the nitrogen atmosphere is not just a safety measure; it is the chemical control agent that dictates the purity and structure of your final carbon material.
Summary Table:
| Feature | Role of Nitrogen Atmosphere | Impact on Biomass Processing |
|---|---|---|
| Environment | Displaces oxygen and prevents oxidation | Prevents material from burning to ash |
| Chemical Path | Enables controlled pyrolysis | Converts precursors into biochar/nanostructures |
| Structural Integrity | Protects developing carbon lattice | Ensures highly ordered and pure carbon structures |
| Secondary Reaction | Inhibits secondary oxidation | Protects byproducts like iron and carbides |
| Flow Type | Continuous high-purity nitrogen flow | Maintains positive pressure and purges impurities |
Unlock Precision in Carbon Material Synthesis with KINTEK
Don't let oxidation compromise your research. KINTEK provides industry-leading thermal solutions designed for the rigorous demands of biomass pyrolysis and nanomaterial development. Backed by expert R&D and manufacturing, we offer high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, all featuring superior sealing technology for perfect atmosphere control. Whether you need standard lab equipment or a system fully customized for your unique high-temperature needs, our team is ready to support your success.
Ready to elevate your material purity? Contact us today to find your ideal furnace solution!
Visual Guide
References
- Junchao Ren, Qingfa Zhang. All‐Biomass Nanocomposite Films via Facile and Sustainable Design Procedure for Thermal Management and Electromagnetic Interference Shielding. DOI: 10.1002/advs.202510372
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- How does a high-temperature tube furnace contribute to the pore regulation of carbon nanofibers? Precision Engineering
- What are the typical working temperature ranges for lab tube furnaces? Find the Right Furnace for Your Process
- What is the significance of using a tubular furnace in waste salt pyrolysis research? Precision for High-Fidelity Data
- How is a three-zone furnace structured? Unlock Precision Heating for Your Lab
- How does a tube heating furnace facilitate the carbon coating process? Boost Layered Oxide Conductivity
- What industries commonly use tube furnaces? Essential for High-Tech Materials and Electronics
- What role does a tube furnace play in tantalum capacitor recycling? Enhancing Metal Recovery Through Pyrolysis
- What conditions does a tubular reactor provide for catalyst reduction? Master Platinum, Copper, and Nickel Activation



















