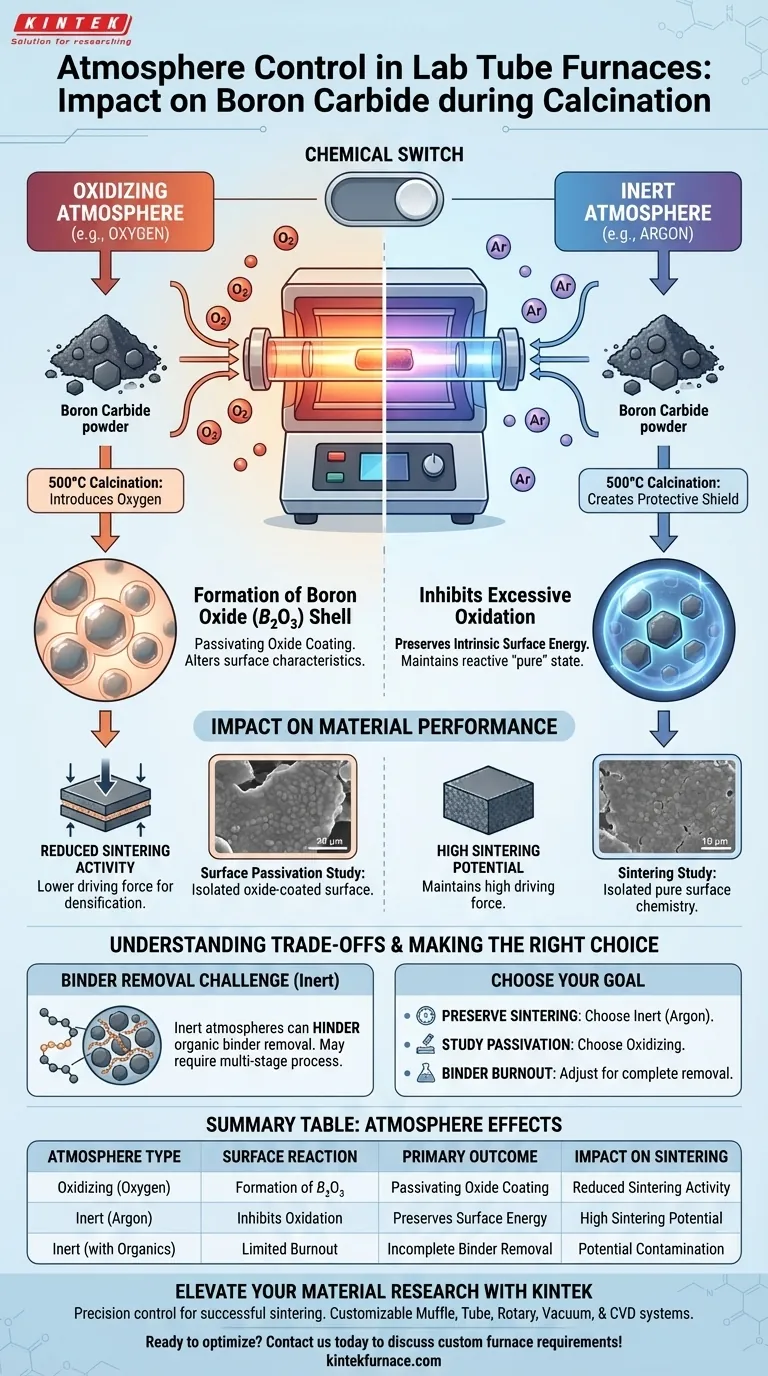
Atmosphere control dictates the surface chemistry of Boron Carbide powders during the 500°C calcination process. When converting hydroxides to oxide coatings, the specific choice of gas within the laboratory tube furnace determines whether the material develops a passivating oxide layer or retains its original surface energy properties.
The selection of an oxidizing versus an inert atmosphere acts as a chemical switch: it either triggers the formation of a Boron Oxide ($B_2O_3$) shell or preserves the high surface energy necessary for subsequent sintering activity.

Mechanisms of Atmosphere Interaction
During the critical 500°C calcination stage, the tube furnace creates a controlled environment that drives specific chemical reactions on the powder's surface.
The Effect of Oxidizing Atmospheres
When oxygen is introduced into the furnace chamber, the Boron Carbide surface reacts chemically.
This reaction results in the formation of a Boron Oxide ($B_2O_3$) layer coating the particles.
This layer alters the fundamental surface characteristics of the powder, effectively changing how it interacts with other materials or how it behaves during later processing steps.
The Role of Inert Atmospheres
Conversely, utilizing an inert atmosphere, such as argon, creates a protective shield around the Boron Carbide.
This environment effectively inhibits excessive oxidation, preventing the formation of the $B_2O_3$ layer.
By blocking oxygen access, the furnace preserves the powder's intrinsic surface energy levels, keeping the material in a more reactive or "pure" state.
Implications for Material Performance
The decision to oxidize or protect the powder is not arbitrary; it directly impacts the material's future behavior.
Impact on Sintering Activity
The primary reference highlights that atmosphere control is critical for studying sintering activity.
Sintering relies heavily on surface energy to drive the densification process.
By using argon to protect surface energy levels, researchers can maintain the high driving force required for effective sintering in subsequent heating stages.
Surface Chemical Characteristics
The tube furnace allows researchers to isolate specific variables regarding surface chemistry.
By controlling the atmosphere, one can precisely study the difference between the oxide-coated surface and the pure Boron Carbide surface.
This capability provides essential data on how surface modifications affect the final performance of the ceramic.
Understanding the Trade-offs
While the primary focus for Boron Carbide at 500°C is surface preservation versus oxidation, using a tube furnace involves balancing multiple process requirements.
Binder Removal vs. Oxidation Protection
It is important to note that while inert atmospheres protect the ceramic surface, they may hinder other processes.
As noted in broader contexts (such as with CGGG materials), oxygen flows are often vital for the complete removal of organic binders like cellulose and glycerol.
Therefore, using a purely inert atmosphere to protect Boron Carbide might complicate the removal of organic additives if they are present in the precursor mix.
Making the Right Choice for Your Goal
The correct atmosphere setting depends entirely on what you are trying to achieve with the Boron Carbide powder.
- If your primary focus is preserving sintering potential: Choose an inert atmosphere (Argon) to inhibit oxidation and maintain high surface energy levels.
- If your primary focus is studying surface passivation: Choose an oxidizing atmosphere to intentionally form a Boron Oxide ($B_2O_3$) layer and analyze its effects.
- If your primary focus is binder burnout: Be aware that a strictly inert atmosphere may require adjustment or a multi-stage process to ensure organics are fully removed without over-oxidizing the powder.
Ultimately, precise atmosphere control transforms the tube furnace from a simple heater into a precision tool for surface engineering.
Summary Table:
| Atmosphere Type | Surface Reaction | Primary Outcome | Impact on Sintering |
|---|---|---|---|
| Oxidizing (Oxygen) | Formation of $B_2O_3$ | Passivating Oxide Coating | Reduced Sintering Activity |
| Inert (Argon) | Inhibits Oxidation | Preserves Surface Energy | High Sintering Potential |
| Inert (with Organics) | Limited Burnout | Incomplete Binder Removal | Potential Contamination |
Elevate Your Material Research with KINTEK
Precision atmosphere control is the difference between a successful sintering process and a failed experiment. KINTEK provides high-performance laboratory solutions tailored for advanced material science.
Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, and CVD systems, all customizable to your unique thermal processing needs. Whether you require precise gas flow for Boron Carbide oxidation or ultra-pure inert environments for surface preservation, our furnaces deliver the reliability your research demands.
Ready to optimize your calcination and sintering workflows? Contact us today to discuss your custom furnace requirements with our technical experts!
Visual Guide
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- Why is a horizontal electric furnace ideal for small-diameter samples? Achieve Superior Uniform Heating
- What role does a high-temperature tube furnace play in the synthesis of SiC nanofibers? Precision CVD Growth at 1100°C
- What is the function of a high-temperature tube furnace? Master High-Entropy Metal Phosphide Synthesis
- What is the role of programmed temperature control in a tube furnace? Optimize N-GC-X Catalyst Synthesis
- How does a tube furnace facilitate the growth of controlled oxide layers on X70 carbon steel? Engineering Precision
- What is the purpose of using a high-temperature tube sintering furnace for selenization? Optimize PC-CNT Porosity
- What are the current market trends for 70mm tube furnaces? Discover Key Drivers in Automation and High-Tech Applications
- What is the primary function of a tube furnace in the pyrolysis of biomass? Achieve Precision in Material Research



















