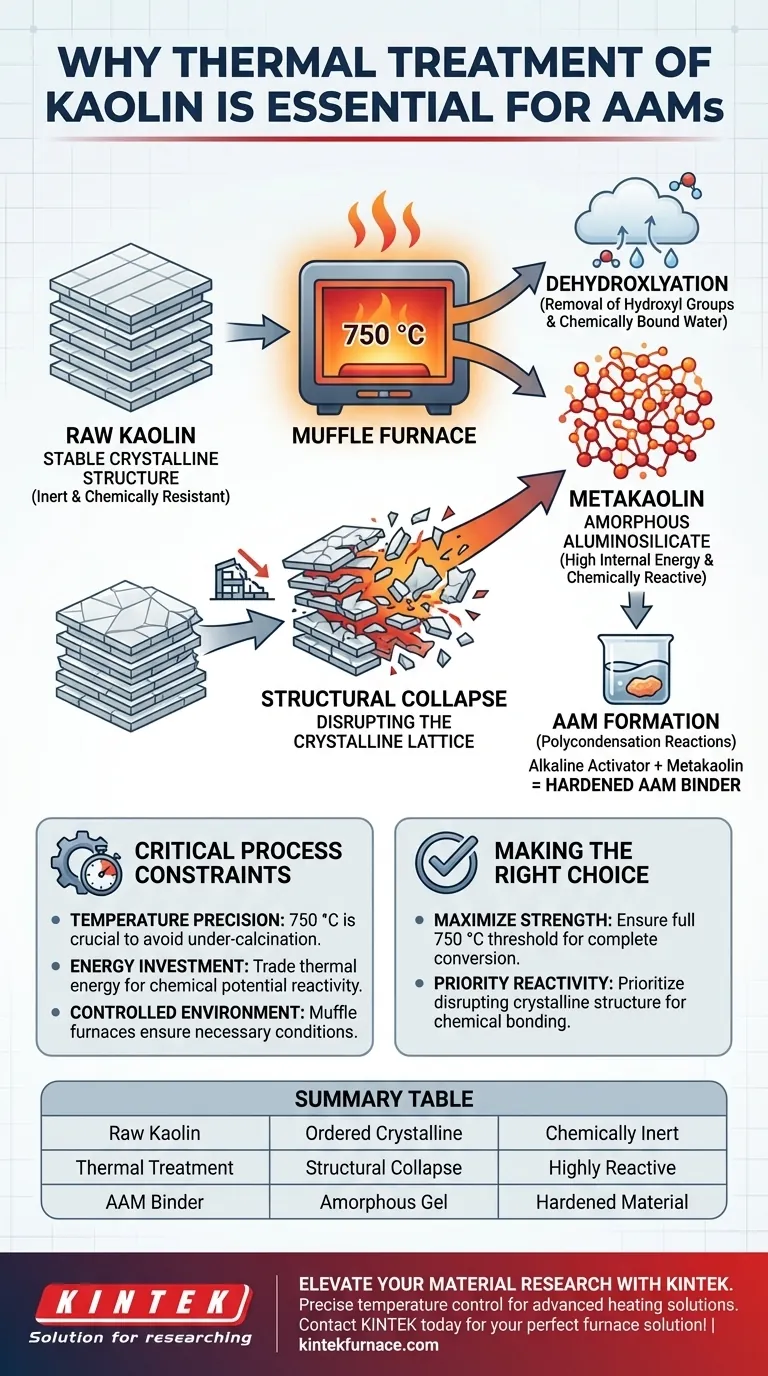
Thermal treatment is the fundamental activation step required to convert inert kaolin clay into a usable precursor for Alkali-Activated Materials (AAMs). By subjecting kaolin to high temperatures in a muffle furnace—typically around 750 °C—you actively strip away chemically bound water and dismantle the clay's stable internal structure. This process yields metakaolin, a highly reactive amorphous material capable of the chemical bonding necessary to form a hardened binder.
Raw kaolin is naturally stable and crystalline, making it chemically resistant. Thermal treatment is required to "break" this stability, transforming the material into a disordered state that is primed to react with alkaline agents.

The Mechanism of Structural Transformation
To understand why thermal treatment is non-negotiable, you must look at how heat alters the atomic architecture of the clay.
Dehydration and Dehydroxylation
The primary function of the muffle furnace is to drive off water.
At high temperatures, kaolin undergoes dehydroxylation, where hydroxyl groups are removed from the clay minerals.
This is not merely drying; it is a chemical modification that permanently alters the composition of the material.
Disrupting the Crystalline Lattice
Raw kaolin possesses a layered, crystalline structure.
This ordered structure is thermodynamically stable, meaning it resists chemical attack and will not react readily with other substances.
Thermal treatment at 750 °C acts as a disruptive force, collapsing these ordered layers and leaving the atomic structure in a chaotic, disordered state.
Creating an Amorphous Aluminosilicate
The result of this structural collapse is the formation of metakaolin.
Metakaolin is an amorphous aluminosilicate, meaning its atoms are not arranged in a rigid, repeating pattern.
This lack of order creates high internal energy and chemical instability, which is the "fuel" for future reactions.
Enabling Polycondensation
The ultimate goal of preparing AAMs is to trigger polycondensation reactions.
These reactions occur when the aluminosilicate source dissolves in an alkaline activator and re-precipitates as a hard gel.
Without thermal treatment, the crystalline kaolin would remain inert, failing to dissolve or react, rendering the production of AAMs impossible.
Critical Process Constraints
While thermal treatment is essential, it introduces specific processing requirements that act as trade-offs against the simplicity of using raw materials.
Dependence on Temperature Precision
The conversion process relies heavily on achieving a specific temperature range, cited as 750 °C.
Failing to reach this temperature results in under-calcination, leaving a portion of the material crystalline and unreactive.
This necessitates the use of controlled environments, like muffle furnaces, rather than open-air firing or lower-temperature drying methods.
Energy Investment for Reactivity
You are effectively trading thermal energy for chemical potential energy.
The process transforms a low-energy, stable material into a high-energy, reactive one.
This makes the production of metakaolin more energy-intensive than using untreated fillers, but it is the only way to impart binding properties to the clay.
Making the Right Choice for Your Goal
The thermal treatment of kaolin is not a variable you can adjust arbitrarily; it is a binary requirement for chemical functionality.
- If your primary focus is maximizing material strength: Ensure your thermal treatment reaches the full 750 °C threshold to guarantee complete conversion to the amorphous state.
- If your primary focus is chemical reactivity: Prioritize the disruption of the crystalline structure, as any remaining crystallinity will act as an inert filler rather than a binder.
Successful Alkali-Activated Materials depend entirely on the quality of the amorphous phase generated during this critical heating stage.
Summary Table:
| Process Phase | Temperature | Structural Effect | Material Outcome |
|---|---|---|---|
| Raw Kaolin | Ambient | Ordered Crystalline Lattice | Chemically Inert / Stable |
| Dehydroxylation | ~750 °C | Removal of Hydroxyl Groups | Loss of Chemically Bound Water |
| Metakaolin | High Heat | Amorphous Structural Collapse | Highly Reactive Binder |
| Polycondensation | Post-Treatment | Dissolution in Alkaline Activator | Hardened AAM Binder |
Elevate Your Material Research with KINTEK
Precise temperature control is the difference between inert clay and a high-performance binder. KINTEK provides the advanced heating solutions required to master the dehydroxylation of kaolin and other critical thermal processes.
Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of laboratory equipment including:
- High-Precision Muffle & Tube Furnaces
- Rotary & Vacuum Systems
- CVD Systems & Customizable High-Temp Solutions
Whether you are developing next-generation Alkali-Activated Materials or scaling industrial ceramics, our customizable systems are engineered to meet your unique research needs.
Ready to optimize your thermal activation? Contact KINTEK today to find your perfect furnace solution!
Visual Guide
References
- Nataša Mladenović Nikolić, Ljiljana Kljajević. Microstructural Analysis and Radiological Characterization of Alkali-Activated Materials Based on Aluminosilicate Waste and Metakaolin. DOI: 10.3390/gels11010057
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What furnace features ensure high-quality firing? Achieve Uniform Sintering & Superior Results
- What are the cost differences between industrial muffle furnaces and drying ovens? Understand the Price Gap and Choose Wisely
- What necessary process conditions does a muffle furnace provide for fruit powder ash analysis? Mastering 550°C Oxidation
- What role does a muffle furnace play in SCS of catalysts? Optimize Thermal Initiation for Manganese-Nickel Synthesis
- How are muffle furnaces categorized based on heating elements? Choose the Right Type for Your Temperature Needs
- How is a muffle furnace utilized in the determination of ash content in biomass samples? Accurate Analysis Guide
- What are the key considerations when selecting a muffle furnace? Ensure Optimal Performance for Your Lab
- How are muffle furnaces used in jewelry making? Achieve Precision in Metal Clay and Annealing



















