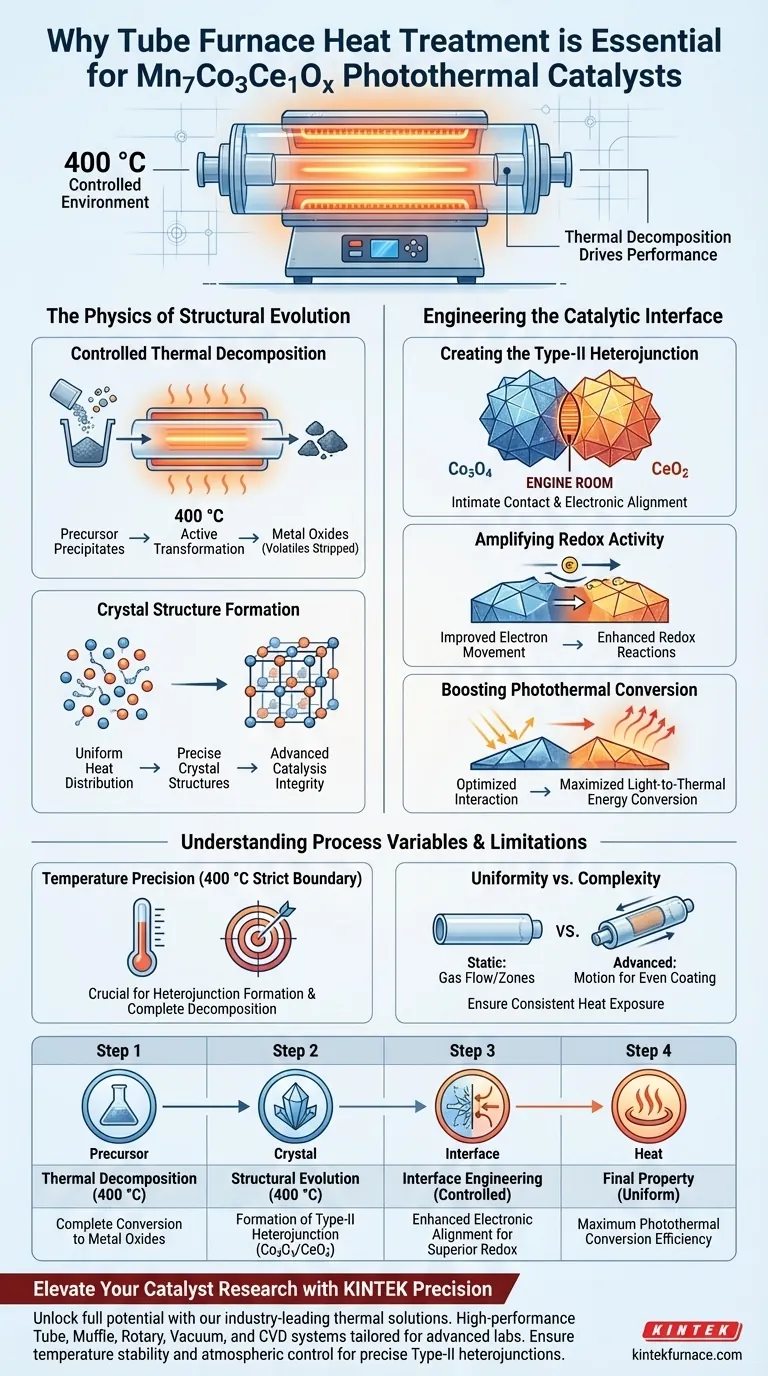
Thermal decomposition drives performance. The heat treatment process using a tube furnace is essential because it provides a strictly controlled environment at 400 °C, forcing precursor precipitates to decompose and reorganize into highly specific composite oxides. This thermal regulation is the primary mechanism that determines the catalyst's final crystal structure and its subsequent efficiency.
The tube furnace does not merely dry the material; it engineers the atomic landscape to form a type-II heterojunction between Co3O4 and CeO2, which is the fundamental driver of the catalyst's enhanced redox activity and photothermal conversion.

The Physics of Structural Evolution
Controlled Thermal Decomposition
At 400 °C, the tube furnace ensures that the precursor materials undergo complete thermal decomposition.
This is not a passive drying phase; it is an active chemical transformation. The heat breaks down the initial precipitates, stripping away volatile components to leave behind the desired metal oxides.
Crystal Structure Formation
The uniform heat distribution within the tube allows these oxides to settle into precise crystal structures.
Without this stable thermal environment, the atoms might arrange chaotically, leading to defects that hinder performance. The tube furnace guarantees the structural integrity required for advanced catalysis.
Engineering the Catalytic Interface
Creating the Type-II Heterojunction
The most critical outcome of this heat treatment is the formation of a type-II heterojunction between Cobalt Oxide (Co3O4) and Cerium Oxide (CeO2).
This interface is the "engine room" of the material. The specific temperature profile of the furnace facilitates the intimate contact and electronic alignment between these two distinct oxides.
Amplifying Redox Activity
Once this heterojunction is established, the material's ability to participate in reduction-oxidation (redox) reactions improves significantly.
The junction promotes better electron movement across the catalyst surface. This directly correlates to the material's effectiveness in photothermal applications.
Boosting Photothermal Conversion
The structural reorganization achieved in the furnace maximizes photothermal conversion efficiency.
By optimizing the interaction between the manganese, cobalt, and cerium components, the material becomes highly efficient at converting light energy into thermal energy, which drives the catalytic process.
Understanding Process Variables and Limitations
The Importance of Temperature Precision
While the tube furnace is powerful, the specific temperature of 400 °C is a strict boundary condition for this specific composite.
Deviating from this temperature can prevent the formation of the necessary heterojunctions or lead to incomplete decomposition. The "controlled" nature of the furnace is just as important as the heat itself.
Uniformity vs. Complexity
Standard tube furnaces provide excellent temperature control, but achieving uniformity across large batches can be challenging.
While some advanced setups (like rotary tube furnaces) use motion to ensure every particle is coated or heated evenly, standard static tube furnaces rely heavily on gas flow and precise heating zones. You must ensure your loading configuration allows for consistent heat exposure to avoid heterogeneous results.
Making the Right Choice for Your Goal
To maximize the potential of your Mn7Co3Ce1Ox catalyst, you must align your heat treatment strategy with your specific performance metrics.
- If your primary focus is Redox Activity: Prioritize the precision of the 400 °C setpoint to guarantee the complete formation of the Co3O4/CeO2 type-II heterojunction.
- If your primary focus is Batch Consistency: Ensure the sample load within the tube allows for uniform heat penetration, or consider agitation methods to prevent thermal gradients.
Precise thermal management is not just a preparation step; it is the architect of your catalyst's functional identity.
Summary Table:
| Process Mechanism | Temperature | Key Outcome |
|---|---|---|
| Thermal Decomposition | 400 °C | Complete conversion of precursor precipitates into metal oxides. |
| Structural Evolution | 400 °C | Formation of a Type-II heterojunction between Co3O4 and CeO2. |
| Interface Engineering | Controlled | Enhanced electronic alignment for superior redox activity. |
| Final Property | Uniform | Maximum photothermal conversion efficiency for light-to-heat energy. |
Elevate Your Catalyst Research with KINTEK Precision
Unlock the full potential of your photothermal materials with KINTEK’s industry-leading thermal solutions. Backed by expert R&D and world-class manufacturing, we provide high-performance Tube, Muffle, Rotary, Vacuum, and CVD systems tailored specifically for advanced lab requirements. Whether you are engineering precise Type-II heterojunctions or requiring uniform Mn7Co3Ce1Ox decomposition, our customizable furnaces ensure the temperature stability and atmospheric control your research demands.
Ready to optimize your catalytic efficiency? Contact our technical experts today to find the perfect high-temperature system for your unique laboratory needs.
Visual Guide
References
- Niansi Li, Qiliang Wang. A Multifunctional Photothermal Catalyst Enabling Full‐Day Sustainable Power and Indoor Air Quality Control. DOI: 10.1002/advs.202505059
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- What are the key factors affecting temperature control in split tube furnaces? Ensure Precision and Uniformity
- Why is a high-temperature tube furnace required for the activation of nitro-functionalized catalysts? (ACN Mastery)
- Why is a horizontal tube furnace utilized for BPEA growth? Mastering Physical Vapor Transport for Single Crystals
- What process conditions does a tube furnace provide for Au-Ni-TiO2 nanowires? Master 1000°C VLS Synthesis
- How does the mature technology of a tube furnace benefit its operation? Achieve Reliable, Cost-Effective Heat Processing
- How does sample handling differ between vertical and horizontal tube furnaces? Choose the Right Furnace for Your Lab
- How does the amount of material processed vary between batch and continuous rotary tube furnaces? Scale Your Production Efficiently
- How does the working temperature range affect the choice of a vertical tube furnace? Optimize Your Lab's Performance and Budget



















