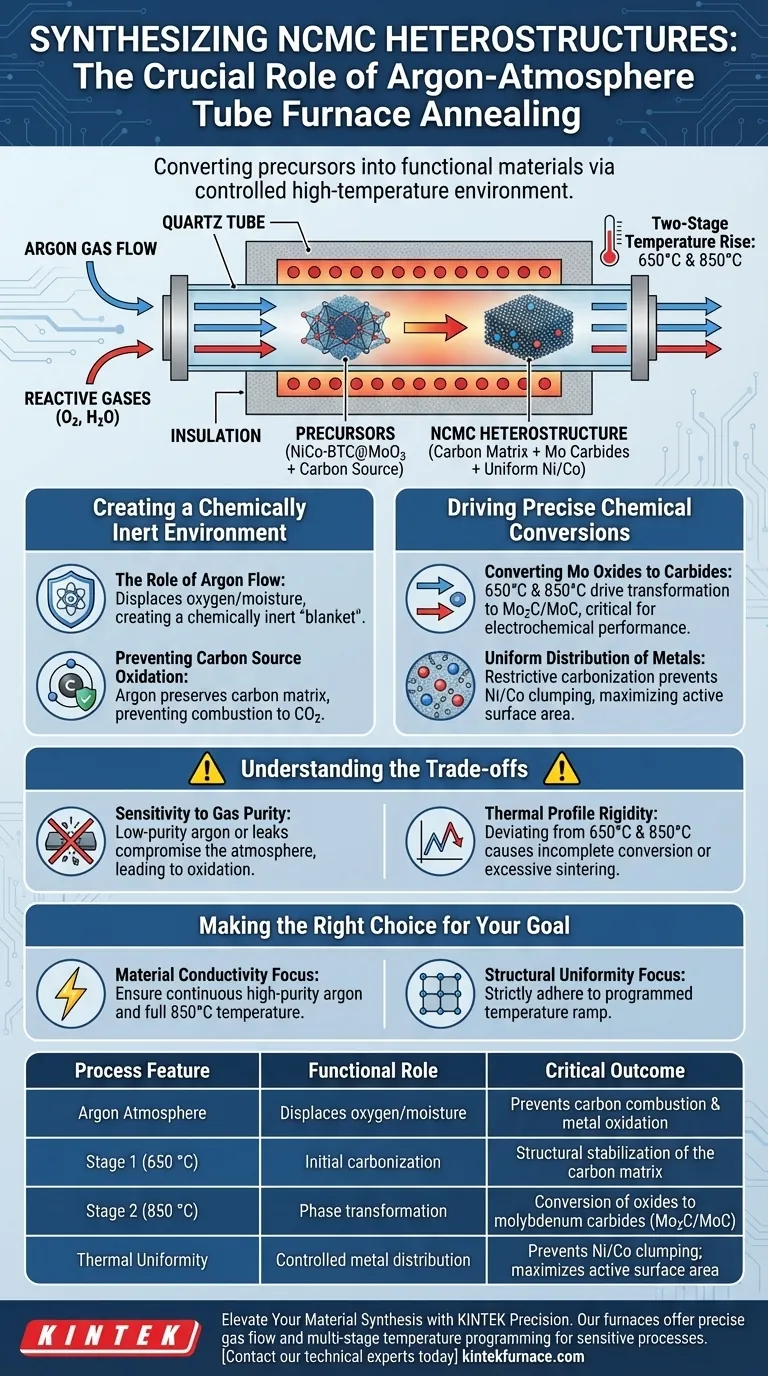
The annealing process in a tube furnace under an argon atmosphere is essential for synthesizing NCMC heterostructures because it creates the strictly controlled environment required to convert precursors into functional materials without degrading them. By maintaining an inert atmosphere during a two-stage temperature rise (at 650 °C and 850 °C), the process drives the conversion of molybdenum oxides into molybdenum carbides while simultaneously preventing the destruction of the carbon source and oxidation of metallic components.
The tube furnace acts as a protective reactor that allows for high-temperature carbonization and phase transformation. Without the argon shield, the carbon matrix would oxidize and burn away, and the precise chemical reduction required to form conductive carbides would fail.

Creating a Chemically Inert Environment
The Role of Argon Flow
The primary function of the argon atmosphere is to displace reactive gases from the furnace chamber.
By removing oxygen and moisture, the argon flow creates a chemically inert "blanket" around the material. This ensures that the chemical reactions occurring inside are driven solely by the thermal energy and the precursor materials, rather than by atmospheric contaminants.
Preventing Carbon Source Oxidation
NCMC heterostructures rely heavily on a conductive carbon matrix.
In the presence of oxygen, high temperatures would cause the carbon source to combust, effectively disappearing as carbon dioxide. The argon atmosphere preserves the carbon, allowing it to form the structural backbone of the composite.
Driving Precise Chemical Conversions
Converting Molybdenum Oxides to Carbides
The tube furnace allows for a specific, programmed temperature rise that facilitates complex phase changes.
Specifically, the heat treatment converts molybdenum oxides from the precursor (NiCo-BTC@MoO3) into molybdenum carbides (Mo2C/MoC). This transformation is critical for the electrochemical performance of the final heterostructure.
Uniform Distribution of Metals
Beyond simple conversion, the process controls how the metals settle within the structure.
The restrictive carbonization process ensures that metallic Nickel and Cobalt are not clumped randomly. Instead, they become uniformly distributed throughout the conductive carbon matrix, which is vital for maximizing the active surface area of the material.
Understanding the Trade-offs
Sensitivity to Gas Purity
While the tube furnace is effective, the "inert" environment is only as good as the gas supply and the system's seal.
If the argon supply is not high-purity, or if there is a leak in the tube, the protective atmosphere is compromised. Even trace amounts of oxygen at 850 °C can lead to surface oxidation of the nickel and cobalt, degrading the material's conductivity.
Thermal Profile Rigidity
The process relies on a specific two-stage heating profile (650 °C and 850 °C).
This is a rigid requirement. Deviating from these specific set points can result in incomplete conversion (leaving behind oxides) or excessive sintering (reducing surface area), meaning the equipment must have precise thermal regulation.
Making the Right Choice for Your Goal
To ensure the successful synthesis of NCMC heterostructures, apply these principles to your experimental design:
- If your primary focus is material conductivity: Ensure the argon flow is continuous and the temperature reaches the full 850 °C to guarantee complete carbonization and carbide formation.
- If your primary focus is structural uniformity: strictly adhere to the programmed temperature ramp to allow metallic Nickel and Cobalt to disperse evenly without agglomerating.
Success in this synthesis relies not just on high heat, but on the absolute exclusion of oxygen to allow the chemistry of carbon and carbides to take shape.
Summary Table:
| Process Feature | Functional Role | Critical Outcome |
|---|---|---|
| Argon Atmosphere | Displaces oxygen/moisture | Prevents carbon combustion & metal oxidation |
| Stage 1 (650 °C) | Initial carbonization | Structural stabilization of the carbon matrix |
| Stage 2 (850 °C) | Phase transformation | Conversion of oxides to molybdenum carbides (Mo2C/MoC) |
| Thermal Uniformity | Controlled metal distribution | Prevents Ni/Co clumping; maximizes active surface area |
Elevate Your Material Synthesis with KINTEK Precision
Achieving the perfect NCMC heterostructure requires absolute atmospheric control and thermal accuracy. KINTEK provides industry-leading Tube, Vacuum, and CVD systems designed to meet the most rigorous research standards. Backed by expert R&D and specialized manufacturing, our furnaces offer the precise gas flow and multi-stage temperature programming essential for sensitive carbonization and carbide conversion processes.
Ready to optimize your high-temperature lab applications? Contact our technical experts today to explore our customizable furnace solutions tailored to your unique research needs.
Visual Guide
References
- Muhammad Ahsan Naseeb, Amir Waseem. Molybdenum carbide supported metal–organic framework-derived Ni, Co phosphosulphide heterostructures as efficient OER and HER catalysts. DOI: 10.1039/d5na00510h
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- How is a high-temperature tube furnace utilized in vertical silicon transistor fabrication? Master Precision Oxidation
- What is the primary role of a tube furnace in CuGaO2 treatment? Enhance Crystallization and Film Performance
- What are the application areas of a 70mm tube furnace? Precision Heating for Materials Science and More
- What core functions does a tube high-temperature furnace perform? Mastering In-situ Carbothermal Reduction
- What benefits does a horizontal tube furnace offer? Achieve Precise Heat Control and Easy Access for Your Lab
- Why is a tube furnace with high-precision control required for annealing platinum-decorated ruthenium catalysts?
- How does a horizontal electric furnace ensure precise thermal control? Achieve Superior Temperature Stability for Your Lab
- Why is uniform heating important in tubular furnaces? Ensure Process Reliability and Predictable Results



















