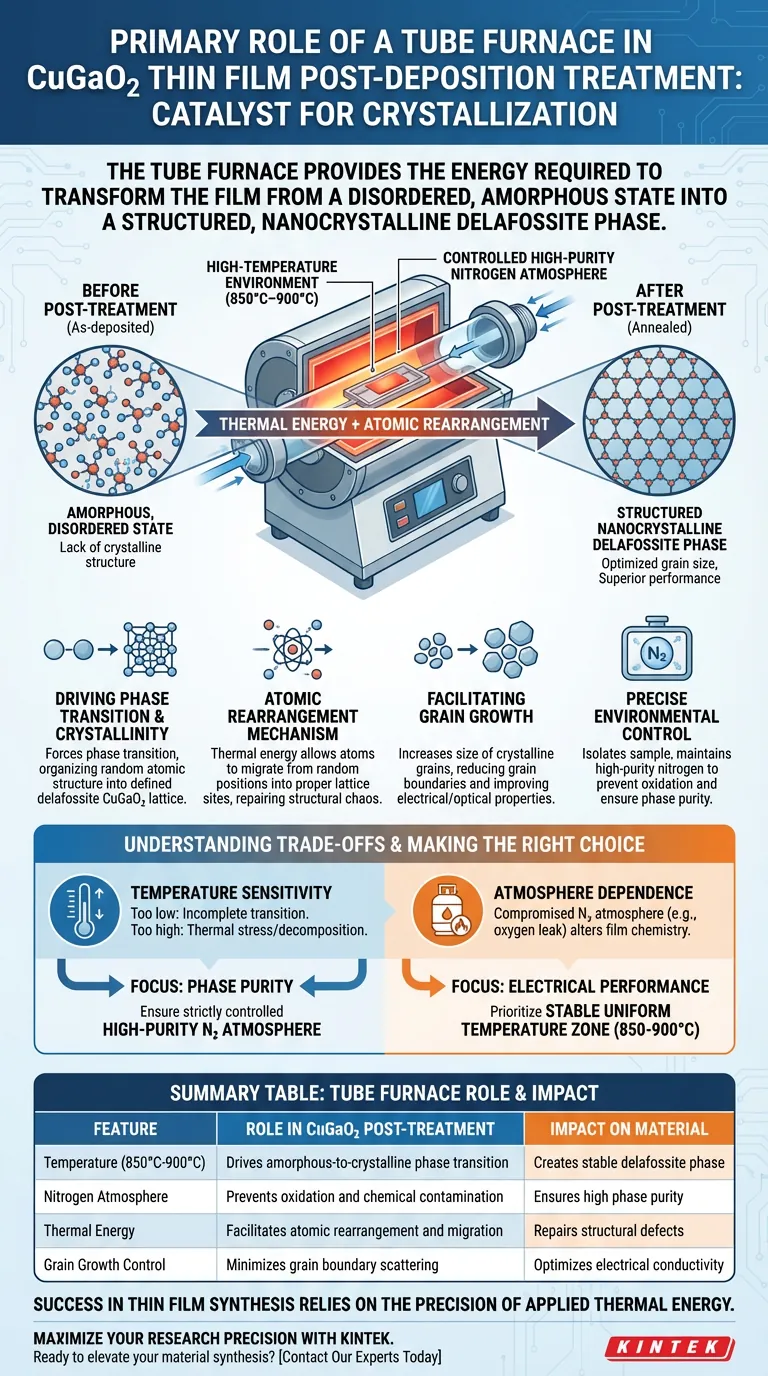
The primary role of a tube furnace in this context is to act as a catalyst for crystallization.
For CuGaO2 thin films specifically, the furnace provides a stable high-temperature environment (typically between 850°C and 900°C) paired with a controlled high-purity nitrogen atmosphere. This precise thermal treatment provides the energy required to transform the film from a disordered, amorphous state into a structured, nanocrystalline delafossite phase.
Core Takeaway Thin films deposited at room temperature often lack the necessary crystalline structure to function effectively. The tube furnace solves this by supplying the thermal energy needed for atomic rearrangement, converting the material into a stable delafossite phase while optimizing its grain size for superior electrical and optical performance.

Driving Phase Transition and Crystallinity
Moving from Amorphous to Structured
When CuGaO2 films are initially deposited at room temperature, their atomic structure is usually amorphous (disordered).
The tube furnace serves as the corrective mechanism. By elevating the temperature to the 850°C–900°C range, it forces a phase transition, organizing the random atomic structure into a defined delafossite CuGaO2 lattice.
The Mechanism of Atomic Rearrangement
Heat is effectively kinetic energy on an atomic scale.
The tube furnace provides the thermal energy required for atoms within the thin film to migrate. This migration allows atoms to move from random positions into their proper lattice sites, repairing the structural chaos inherent in the as-deposited film.
Optimizing Material Properties
Facilitating Grain Growth
Beyond simple crystallization, the tube furnace is critical for increasing the size of the crystalline grains.
Larger grains generally result in fewer grain boundaries, which are barriers to electron flow. By promoting grain growth, the annealing process directly improves the electrical conductivity and optical characteristics of the CuGaO2 film.
Precise Environmental Control
The "tube" design of the furnace allows for the isolation of the sample from the outside atmosphere.
For CuGaO2, maintaining a high-purity nitrogen atmosphere is essential. This controlled environment prevents unwanted oxidation or chemical reactions that would occur in standard air, ensuring the purity of the final phase.
Understanding the Trade-offs
Temperature Sensitivity
While high heat is necessary, it is a double-edged sword.
If the temperature is too low, the amorphous-to-crystalline transition will remain incomplete, leaving the material with poor properties. Conversely, excessive temperatures can lead to thermal stress or unwanted decomposition of the film components.
Atmosphere Dependence
The success of the tube furnace treatment relies heavily on the integrity of the gas flow.
Even with the correct temperature, a compromise in the nitrogen atmosphere (such as a leak introducing oxygen) can fundamentally alter the chemistry of the film. You rely entirely on the furnace's ability to maintain a sealed, positive-pressure environment to achieve the delafossite phase.
Making the Right Choice for Your Goal
To maximize the effectiveness of post-deposition annealing for CuGaO2, align your furnace parameters with your specific material objectives:
- If your primary focus is Phase Purity: Ensure your tube furnace is capable of maintaining a strictly controlled high-purity nitrogen atmosphere to prevent surface oxidation during the high-temperature dwell time.
- If your primary focus is Electrical Performance: Prioritize a furnace with a stable uniform temperature zone at 850°C–900°C to maximize grain growth and minimize grain boundary scattering.
Success in thin film synthesis relies not just on deposition, but on the precision of the thermal energy applied afterward.
Summary Table:
| Feature | Role in CuGaO2 Post-Treatment | Impact on Material |
|---|---|---|
| Temperature (850°C-900°C) | Drives amorphous-to-crystalline phase transition | Creates stable delafossite phase |
| Nitrogen Atmosphere | Prevents oxidation and chemical contamination | Ensures high phase purity |
| Thermal Energy | Facilitates atomic rearrangement and migration | Repairs structural defects |
| Grain Growth Control | Minimizes grain boundary scattering | Optimizes electrical conductivity |
Maximize Your Research Precision with KINTEK
Achieving the perfect delafossite phase requires absolute control over thermal uniformity and atmospheric purity. Backed by expert R&D and manufacturing, KINTEK offers high-performance Tube, Muffle, Rotary, Vacuum, and CVD systems designed for the most demanding thin-film applications. Our systems are fully customizable to meet your unique temperature and gas-flow requirements, ensuring your CuGaO2 films achieve superior crystallinity and electrical performance.
Ready to elevate your material synthesis?
Contact Our Experts Today" Form)"
Visual Guide
References
- Akash Hari Bharath, Kalpathy B. Sundaram. Deposition and Optical Characterization of Sputter Deposited p-Type Delafossite CuGaO2 Thin Films Using Cu2O and Ga2O3 Targets. DOI: 10.3390/ma17071609
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Inclined Rotary Plasma Enhanced Chemical Deposition PECVD Tube Furnace Machine
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What role does a high-temperature tube furnace play in Mo2C synthesis? Master Carbonization Precision
- What are the options for zonal heating in horizontal tube furnaces? Optimize Your Thermal Control
- What is the purpose of performing thermal annealing in vacuum-sealed glass tubes for nickel oxide films?
- What is the working environment of a vacuum tube furnace? Achieve Purity and Precision in Material Processing
- What features ensure precise temperature control in tube furnaces? Discover the Key Components for Accuracy
- How do Quartz Tube Furnaces support controlled atmosphere experiments? Master Precise Material Synthesis
- Why is the space-saving design of a tube furnace advantageous? Unlock Efficiency in Your Lab
- What are the specific operational benefits of tube furnace cracking? Unlock Efficiency and Precision in Material Processing



















