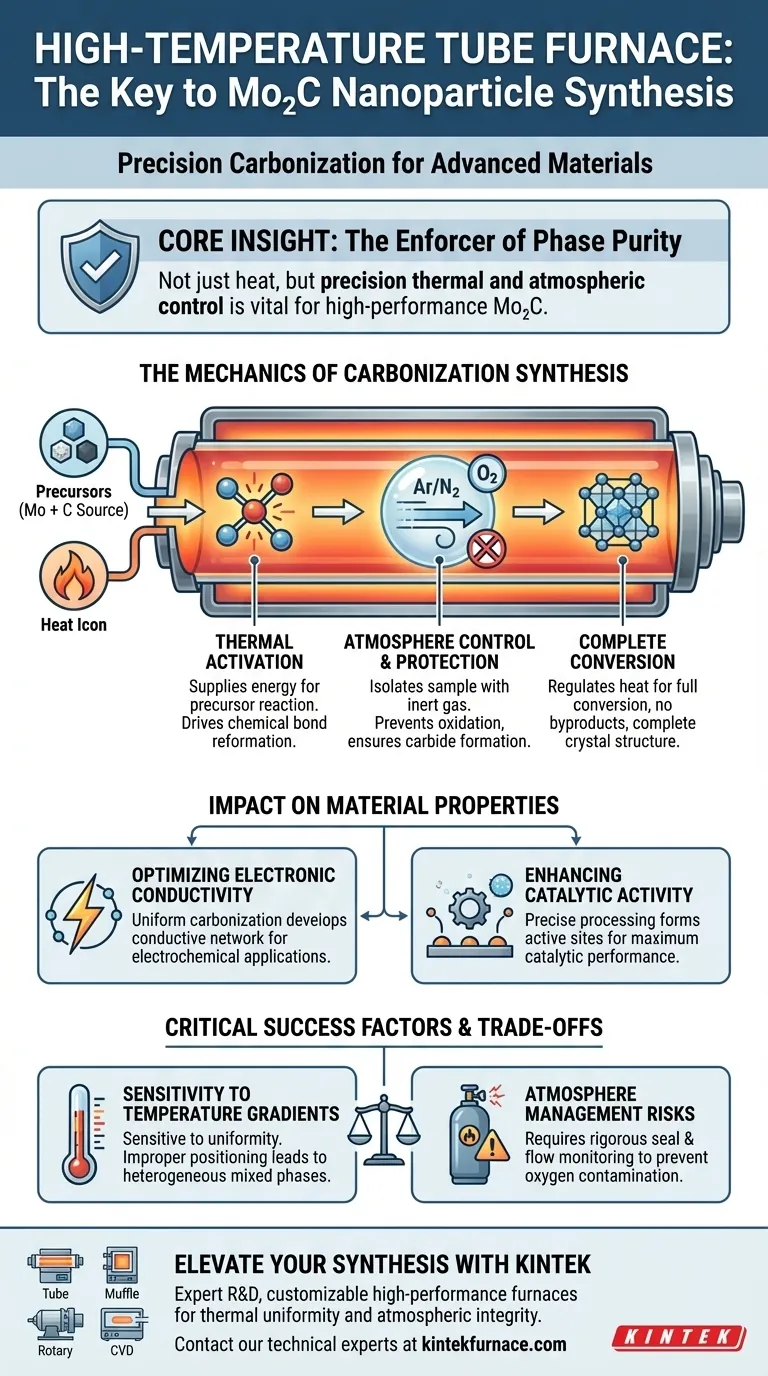
The primary role of a high-temperature tube furnace in this process is to provide a strictly controlled thermal environment that drives the chemical reaction between a molybdenum source (such as ammonium molybdate) and a carbon source (such as urea). By maintaining a precise high-temperature setting under an inert or reducing atmosphere, the furnace facilitates the complete conversion of these precursors into molybdenum carbide (Mo2C) nanoparticles.
Core Insight: The tube furnace is not merely a heat source; it is a precision instrument used to enforce phase purity. Its ability to maintain thermal uniformity and specific atmospheric conditions is the deciding factor in creating nanoparticles with the high electronic conductivity and catalytic activity required for advanced applications.

The Mechanics of Carbonization Synthesis
Thermal Activation of Precursors
The synthesis of Mo2C requires significant thermal activation energy to initiate the reaction between the metal and carbon sources. The tube furnace supplies this energy, heating the ammonium molybdate and urea mixture to the specific point where chemical bonds break and reform. This thermal treatment drives the breakdown of the organic carbon source and the subsequent carburization of the molybdenum.
Atmosphere Control and Protection
A critical function of the tube furnace is its ability to isolate the sample from the ambient environment. The design allows for the introduction of inert or reducing gases, creating a specific atmosphere within the tube. This prevents oxygen from interfering with the reaction, ensuring that the molybdenum forms a carbide (Mo2C) rather than an unwanted oxide.
Ensuring Complete Conversion
To achieve high-performance nanoparticles, the precursors must be fully converted without leaving unreacted byproducts. The precise temperature control systems within the furnace regulate the heat to ensuring the reaction proceeds to completion. This results in a final product characterized by a complete crystal structure and high chemical stability.
Impact on Material Properties
Optimizing Electronic Conductivity
The quality of the thermal treatment directly influences the electronic properties of the final material. By ensuring a uniform and complete carbonization process, the furnace helps develop the material's conductive network. This results in Mo2C nanoparticles that exhibit the high electronic conductivity essential for electrochemical applications.
Enhancing Catalytic Activity
The performance of Mo2C as a catalyst is heavily dependent on the surface properties developed during synthesis. The controlled environment of the tube furnace allows for the accurate formation of active sites. This precise processing ensures the nanoparticles achieve maximum catalytic activity, making them effective for their intended chemical reactions.
Understanding the Trade-offs
Sensitivity to Temperature Gradients
While tube furnaces offer excellent control, the synthesis is highly sensitive to temperature uniformity. If the "hot zone" within the tube varies significantly, it can lead to heterogeneous products with mixed phases. It is vital to position the sample correctly within the uniform temperature zone to avoid incomplete carbonization.
Atmosphere Management Risks
The dependence on a controlled atmosphere introduces a risk regarding gas flow and seal integrity. Even minor leaks or insufficient gas flow rates can introduce oxygen, compromising the purity of the Mo2C. The process requires rigorous monitoring of gas inputs to maintain the strictly inert or reducing environment necessary for carbide formation.
Making the Right Choice for Your Goal
To maximize the quality of your molybdenum carbide nanoparticles, tailor your furnace parameters to your specific objectives:
- If your primary focus is Phase Purity: Ensure your sample is positioned strictly within the furnace's uniform temperature zone to guarantee homogenous carbonization across the entire batch.
- If your primary focus is Catalytic Performance: Prioritize precise control over the heating rate and dwell time to optimize the formation of active sites without sintering the particles.
- If your primary focus is Chemical Stability: Verify the integrity of your inert gas flow (Argon or Nitrogen) to completely eliminate oxygen exposure during the high-temperature phase.
The success of Mo2C synthesis relies less on the maximum temperature capable and more on the consistency and atmospheric control the furnace provides throughout the reaction window.
Summary Table:
| Feature | Role in Mo2C Synthesis | Impact on Final Product |
|---|---|---|
| Thermal Activation | Supplies energy for precursor reaction | Drives complete conversion of Mo/Carbon sources |
| Atmosphere Control | Provides inert or reducing environments | Prevents oxidation; ensures phase purity |
| Uniform Heating | Maintains consistent "hot zone" | Eliminates heterogeneous products and mixed phases |
| Process Regulation | Controls heating rate and dwell time | Optimizes catalytic activity and surface active sites |
Elevate Your Nanomaterial Synthesis with KINTEK
Achieving phase-pure molybdenum carbide (Mo2C) requires more than just heat—it demands absolute precision. Backed by expert R&D and manufacturing, KINTEK offers high-performance Tube, Muffle, Rotary, Vacuum, and CVD systems designed to provide the thermal uniformity and atmospheric integrity your research requires.
Whether you are optimizing electronic conductivity or maximizing catalytic activity, our lab high-temp furnaces are fully customizable to meet your unique carbonization needs.
Ready to refine your synthesis process? Contact our technical experts today to find the ideal furnace solution for your laboratory.
Visual Guide
References
- Radha Bhardwaj, Martin Pumera. Laser‐Assisted Mo <sub>2</sub> C‐Derived Patterned Oxide for Highly Selective Room Temperature Ammonia Sensor for Food Spoilage Monitoring. DOI: 10.1002/smtd.202501246
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- How do vertical tube furnaces contribute to advancements in material science and industrial production? Unlock Precision in Material Innovation
- What role does a high-temperature tube furnace play in the solid-state synthesis of LIB cathode materials? Key Insights
- How are wafers loaded and unloaded in a vertical tube furnace? Achieve Precision and Purity in Wafer Processing
- What role does a horizontal tubular furnace play in VACNT synthesis? Master CVD Growth for High-Quality Nanotubes
- What is a laboratory tube furnace and how is it designed? Master Precise Heating for Your Lab
- How do roller kilns and tube furnaces differ in their use of Alumina ceramic tubes? Compare Transport vs. Containment
- What is the role of a tube furnace during fuel cell feasibility studies? Optimize Your Thermal Control
- What role does a programmable tube furnace play in the remelting of cast iron? Expert Insights on Thermal Precision



















