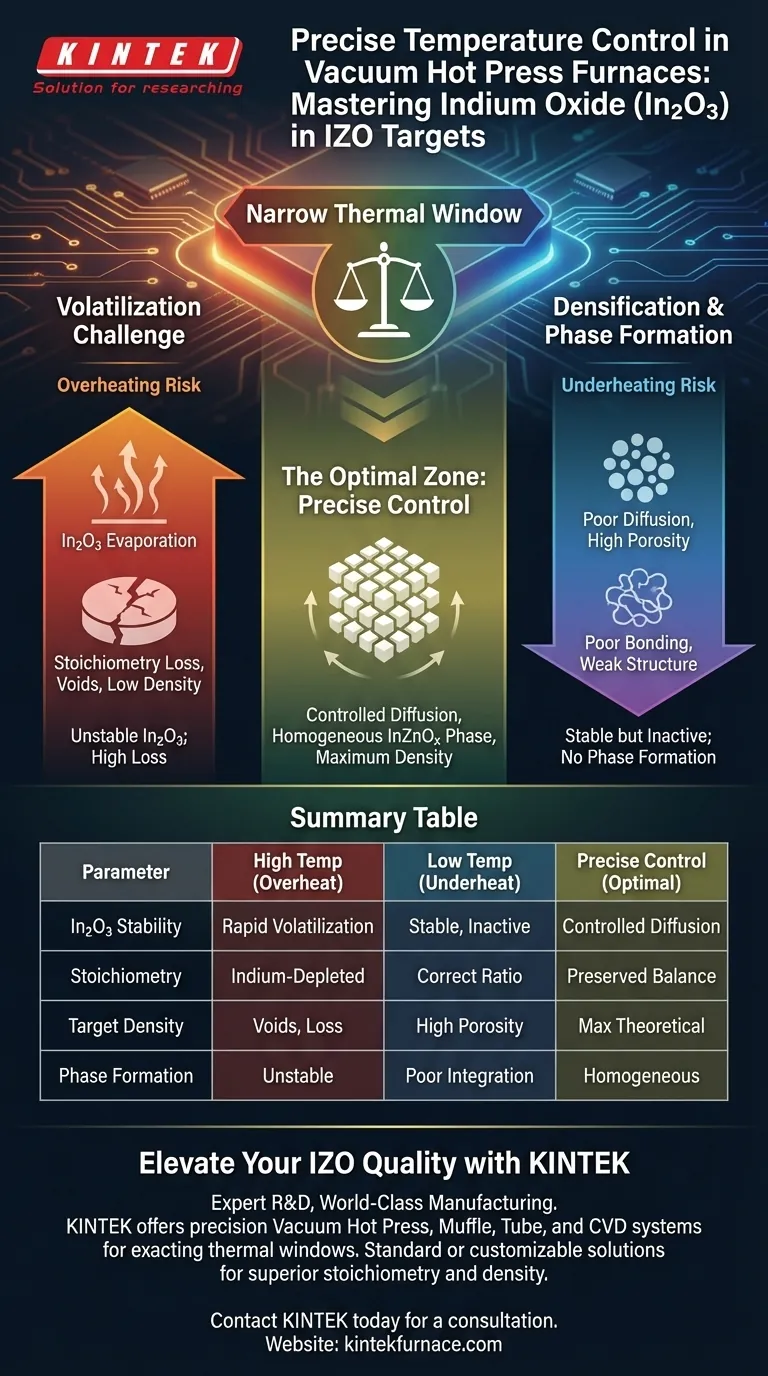
Precise temperature control is the defining factor in preventing the destructive volatilization of Indium Oxide (In2O3) during the manufacturing of IZO targets. Without exacting thermal regulation in a vacuum hot press furnace, the high temperatures required for sintering will cause In2O3 to evaporate, destroying the target’s chemical stoichiometry and compromising its structural density.
Core Takeaway The manufacturing of Indium Zinc Oxide (IZO) targets requires navigating a narrow thermal window. You must apply enough heat to promote the diffusion of Indium into the Zinc Oxide lattice for densification, yet strictly limit that heat to prevent Indium Oxide from vaporizing under vacuum pressure.

The Volatilization Challenge
The Instability of Indium Oxide
Under the conditions of high temperature and low pressure (vacuum), Indium Oxide (In2O3) becomes thermodynamically unstable.
Unlike more robust oxides, In2O3 is highly prone to volatilization. If the temperature creates a vapor pressure that exceeds the vacuum level, the material begins to evaporate rather than sinter.
Consequences for Stoichiometry
When In2O3 volatilizes, it leaves the target matrix. This alters the precise chemical ratio (stoichiometry) between Indium and Zinc.
A loss of Indium results in a target that deviates from its intended electrical and optical properties, rendering it defective for high-precision applications.
Impact on Target Density
Volatilization leaves behind voids where solid material should be. This prevents the target from achieving full theoretical density.
Low-density targets degrade faster during sputtering and produce films with higher particulate contamination.
Achieving Phase Formation
Promoting Atomic Diffusion
While heat carries the risk of volatilization, it is also the catalyst for necessary structural changes.
Precise heat application promotes the diffusion of Indium into the Zinc Oxide lattice. This atomic movement is required to form the specific InZnOx crystalline phases that define the material's properties.
Balancing Densification
The vacuum hot press process relies on the synergy of heat and mechanical pressure.
Temperature control ensures the material reaches a state where pressure can effectively rearrange particles and eliminate pores. If the temperature is too low, the material remains too rigid for the pressure to close internal gaps, resulting in a porous structure.
Understanding the Trade-offs
The Risk of Overheating
If the furnace overshoots the optimal temperature profile, the rate of In2O3 volatilization accelerates exponentially.
This results in a "zinc-rich" target surface with depleted Indium levels and significant mass loss. No amount of mechanical pressure can compensate for the chemical material lost to evaporation.
The Risk of Underheating
Conversely, failing to reach the necessary thermal threshold prevents the formation of the InZnOx phases.
While this preserves the Indium content, it results in a target with poor inter-particle bonding and low density. The target will likely suffer from weak mechanical integrity and inconsistent performance.
Uniformity is Critical
Temperature control is not just about the peak heat; it is about uniformity across the target.
As noted in broader metallurgical applications, uneven heating leads to defects. In IZO targets, a temperature gradient could cause one section to volatilize (lose Indium) while another section remains under-sintered (low density).
Making the Right Choice for Your Process
To optimize your IZO target production, your thermal profiles must reflect your specific quality priorities:
- If your primary focus is Compositional Accuracy: Prioritize strict upper-limit temperature clamps to prevent In2O3 volatilization and preserve stoichiometry.
- If your primary focus is Maximum Density: Focus on extending the dwell time at the highest safe temperature to maximize Indium diffusion into the Zinc lattice without crossing the evaporation threshold.
Success in IZO sintering is ultimately defined by your ability to maintain the target material in the precise zone where densification is active, but volatilization is suppressed.
Summary Table:
| Parameter Impact | High Temperature (Overheating) | Low Temperature (Underheating) | Precise Control (Optimal) |
|---|---|---|---|
| In2O3 Stability | Rapid volatilization/evaporation | Stable but inactive diffusion | Controlled diffusion, no loss |
| Stoichiometry | Indium-depleted (Zinc-rich) | Correct chemical ratio | Preserved chemical balance |
| Target Density | Voids from material loss | High porosity (low bonding) | Maximum theoretical density |
| Phase Formation | Unstable phase distribution | Poor In/Zn lattice integration | Homogeneous InZnOx phase |
Elevate Your IZO Target Quality with KINTEK
Don't let Indium Oxide volatilization compromise your materials. Backed by expert R&D and world-class manufacturing, KINTEK offers precision-engineered Vacuum Hot Press, Muffle, Tube, and CVD systems designed to maintain the exacting thermal windows required for high-purity sintering. Whether you need a standard solution or a fully customizable furnace for unique lab needs, our technology ensures superior stoichiometry and structural density for your target manufacturing.
Ready to optimize your thermal profiles? Contact KINTEK today for a consultation.
Visual Guide
Related Products
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
- 9MPa Air Pressure Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
People Also Ask
- What is a vacuum forming machine used for? A Guide to Cost-Effective Plastic Shaping
- What are the benefits of using a vacuum hot press sintering furnace for the preparation of SiCw/2024 aluminum matrix composites? Achieve High-Performance Aerospace Materials
- What role does a high-pressure press play in the preparation of zinc sample pellets? Optimize Carbothermic Reduction
- What is the purpose of using a hydrogen-argon mixture for hot-pressing SnSe? Enhance Thermoelectric zT Efficiency
- What functions do graphite molds serve in the vacuum hot pressing of copper-carbon nanotube composites?
- What are the advantages of benchtop SPS/FAST for titanium R&D? Accelerate Your Microstructural Engineering
- What are the primary technical advantages of using a Spark Plasma Sintering (SPS) system? Achieve Superior Sintering
- How does a vacuum hot press sintering furnace mitigate copper sintering swelling? Solve Fe-Cu Expansion Issues



















