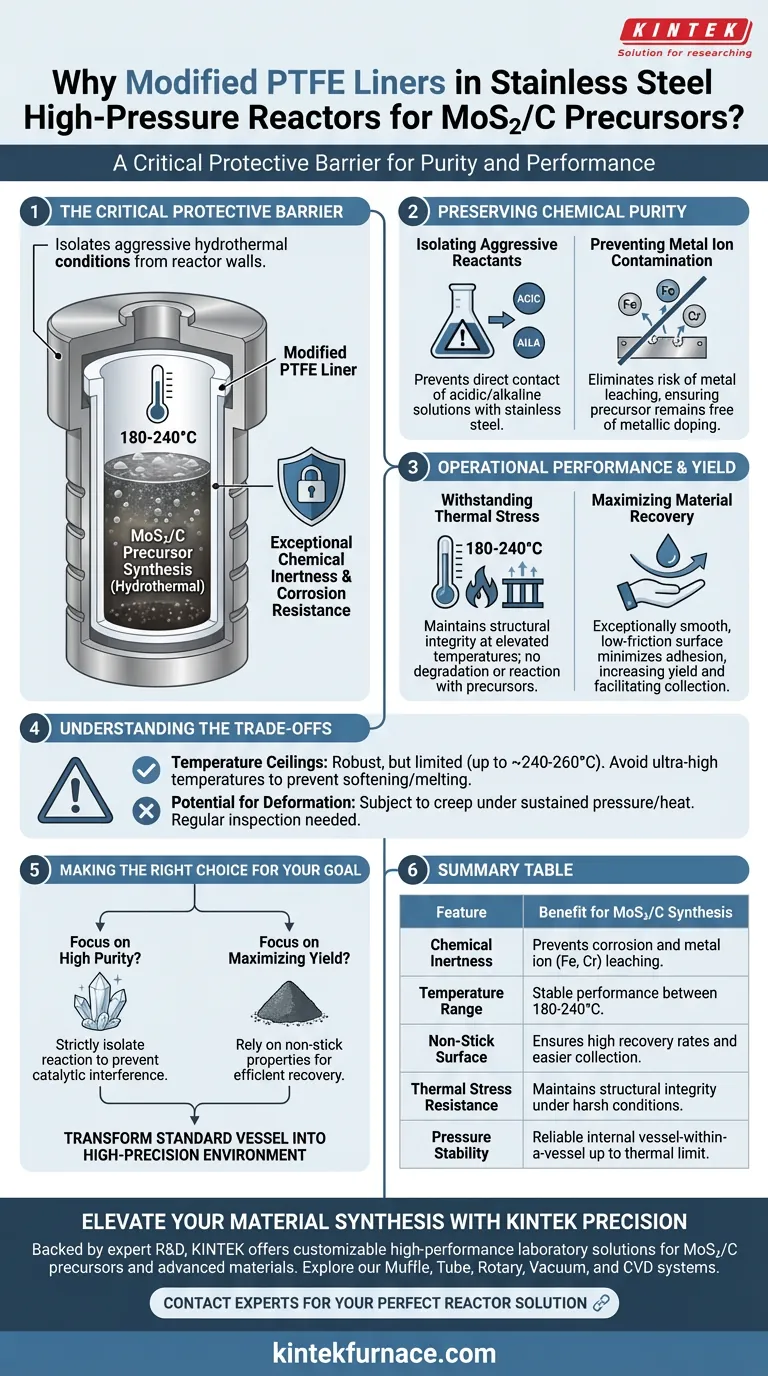
Modified polytetrafluoroethylene (PTFE) acts as a critical protective barrier inside stainless steel high-pressure reactors. Its primary role is to provide exceptional chemical inertness and corrosion resistance during hydrothermal synthesis, specifically within the 180-240°C temperature range, ensuring the purity of the MoS2/C precursor.
Hydrothermal synthesis creates a harsh internal environment; the PTFE liner is essential for preventing metal ion contamination from the reactor walls while ensuring high material recovery rates through its non-stick surface.

Preserving Chemical Purity
Isolating Aggressive Reactants
Hydrothermal synthesis often involves acidic or alkaline solutions under high pressure.
Without a liner, these aggressive reactants would come into direct contact with the stainless steel body.
The PTFE liner effectively creates a chemically inert "vessel within a vessel," shielding the reactor's structural metal from corrosion.
Preventing Metal Ion Contamination
For sensitive applications like MoS2/C precursors, purity is paramount.
If the reaction mixture touches the stainless steel, metal ions (such as iron or chromium) can leach into the solution.
The PTFE liner eliminates this risk, ensuring the final precursor remains free of unintended metallic doping.
Operational Performance and Yield
Withstanding Thermal Stress
Modified PTFE is specifically selected for its ability to maintain structural integrity at elevated temperatures.
It remains stable in hydrothermal environments reaching 180-240°C.
This thermal resistance ensures the liner does not degrade or react with the precursor during the heating phase.
Maximizing Material Recovery
The physical properties of the liner surface are just as important as its chemical properties.
PTFE possesses an exceptionally smooth, low-friction surface.
This minimizes the adhesion of the synthesized precursors to the reactor walls, significantly increasing the yield and making sample collection easier.
Understanding the Trade-offs
Temperature Ceilings
While robust, modified PTFE has a definite thermal limit compared to the steel shell.
Operating significantly above 240-260°C poses a risk of the liner softening, deforming, or melting.
For ultra-high temperature reactions, a PTFE liner is not a suitable solution and alternative materials (like PPL or quartz) must be considered.
Potential for Deformation
PTFE is a polymer and is subject to physical creep under sustained high pressure and temperature.
Over many cycles, the liner may deform slightly, potentially affecting the internal volume or the seal tightness.
Regular inspection of the liner's shape is necessary to ensure safety and consistency.
Making the Right Choice for Your Goal
When designing your synthesis protocol for MoS2/C precursors, consider your specific constraints:
- If your primary focus is high purity: Use the PTFE liner to strictly isolate the reaction from the steel vessel to prevent catalytic interference from leached metal ions.
- If your primary focus is maximizing yield: Rely on the liner’s non-stick properties to ensure valuable nanomaterials are not lost due to wall adhesion.
By functioning as an inert shield, the PTFE liner effectively transforms a standard industrial vessel into a high-precision environment suitable for advanced nanomaterial synthesis.
Summary Table:
| Feature | Benefit for MoS2/C Synthesis |
|---|---|
| Chemical Inertness | Prevents corrosion and metal ion (Fe, Cr) leaching into precursors. |
| Temperature Range | Stable performance during hydrothermal synthesis between 180-240°C. |
| Non-Stick Surface | Ensures high material recovery rates and easier sample collection. |
| Thermal Stress Resistance | Maintains structural integrity under harsh hydrothermal conditions. |
| Pressure Stability | Acts as a reliable internal vessel-within-a-vessel up to its thermal limit. |
Elevate Your Material Synthesis with KINTEK Precision
Precision in nanomaterial synthesis starts with the right equipment. Backed by expert R&D and manufacturing, KINTEK offers high-performance laboratory solutions—including Muffle, Tube, Rotary, Vacuum, and CVD systems—all of which are fully customizable to meet your unique hydrothermal and high-temperature needs. Whether you are producing MoS2/C precursors or advanced ceramics, our equipment ensures maximum purity and process control.
Ready to optimize your lab's efficiency and output? Contact our technical experts today to find your perfect reactor solution!
Visual Guide
References
- One-Pot Hydrothermal Synthesis and Electrochemical Performance of Subspheroidal Core–Shell Structure MoS2/C Composite as Anode Material for Lithium-Ion Batteries. DOI: 10.3390/en17071678
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Molybdenum Disilicide MoSi2 Thermal Heating Elements for Electric Furnace
- Stainless Steel Quick Release Vacuum Chain Three Section Clamp
- Magnesium Extraction and Purification Condensing Tube Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- Why use high-purity graphite for β-Ga2O3 annealing? Key to Thermal Precision & Safety
- What roles do metal shielding disks and heat shields play in in-situ SEM? Ensure Precision & Protect Your Lab Equipment
- What roles do high-purity graphite molds play in the SPS of copper sulfide? Enhance Your Thermoelectric Material Quality
- What makes high-purity alumina crucibles the preferred choice for BZT synthesis? Ensure Purity & Thermal Stability
- Why is it necessary to use high-purity alumina crucibles for sintering hydroxyapatite? Ensure Chemical Phase Purity
- What core environmental protection does an argon-protected glove box provide for sodium-ion batteries? Maximize Safety
- What is the key technological improvement in the circulating water vacuum pump? Discover the Self-Contained Closed-Loop System
- Why are sealed Niobium (Nb) tubes utilized as reaction vessels during the high-temperature solid-state synthesis of Ba1-xEuxZn2Sb2?



















