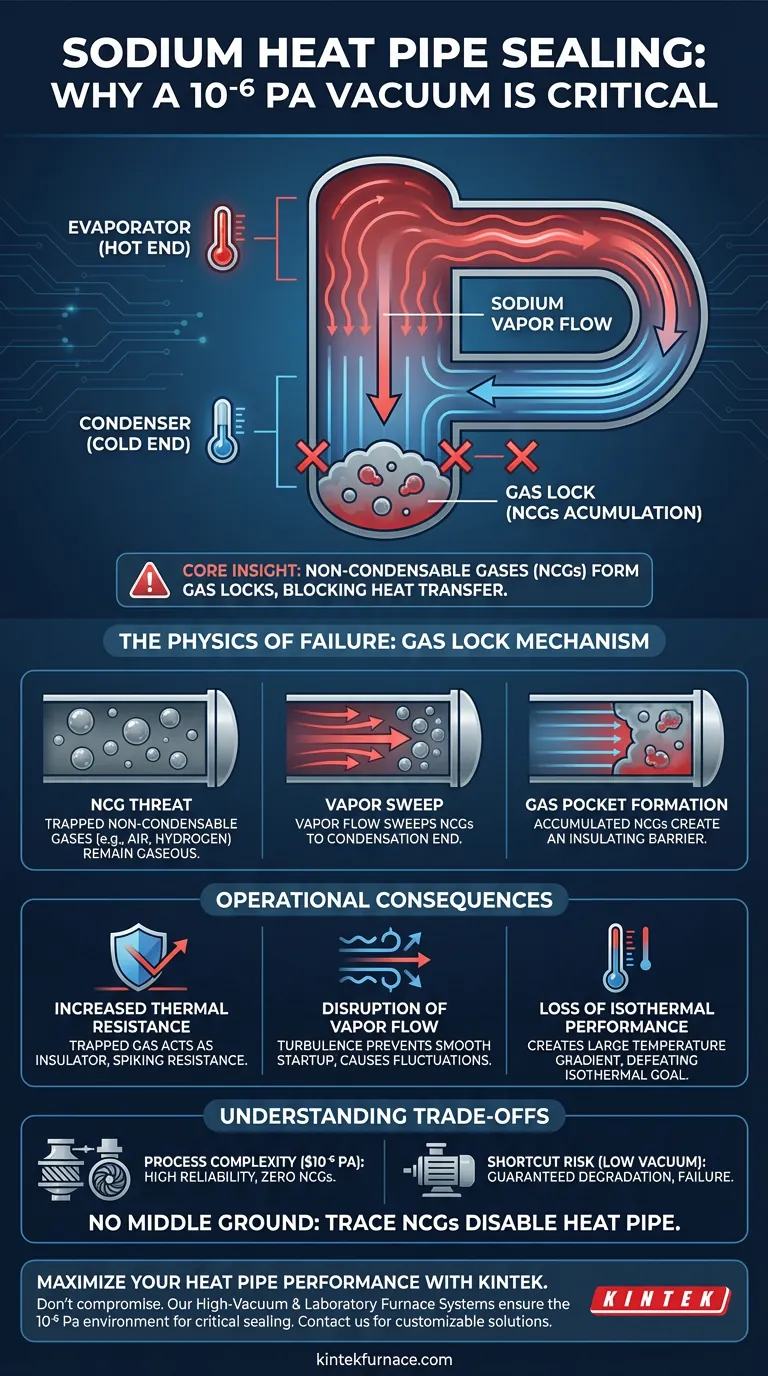
To ensure the functional integrity of a sodium heat pipe, maintaining a vacuum level of $10^{-6}$ Pa during sealing is mandatory. This extreme vacuum is the only reliable method to completely eliminate non-condensable gases from the system. If these gases remain, they disrupt the thermodynamic cycle of the sodium, rendering the heat pipe ineffective.
Core Insight: The requirement for high vacuum is not just about material purity; it is about preventing "gas locks." Any residual non-condensable gases will accumulate at the condensation end of the pipe, creating a barrier that blocks heat transfer and prevents the device from achieving an isothermal state.

The Physics of Heat Pipe Failure
The Threat of Non-Condensable Gases (NCGs)
The primary enemy of a sodium heat pipe is the presence of non-condensable gases (typically air or hydrogen) trapped inside the casing.
Unlike the sodium working fluid, which cycles between liquid and vapor, these gases remain in a gaseous state permanently during operation.
The Mechanism of "Gas Locks"
When the heat pipe operates, the flow of sodium vapor sweeps these non-condensable gases toward the condensation end (the cold end) of the pipe.
Because the gases cannot condense into liquid, they accumulate and form a pocket, or "gas lock."
This pocket effectively reduces the active length of the condenser, physically blocking the sodium vapor from reaching the cooling surface.
Operational Consequences
Increased Thermal Resistance
The immediate result of a gas lock is a significant spike in heat transfer resistance.
Instead of conducting heat efficiently, the trapped gas acts as an insulator at the very point where heat needs to be rejected.
Disruption of Vapor Flow
The presence of NCGs disrupts the smooth, continuous flow of sodium vapor from the evaporator to the condenser.
This turbulence prevents the heat pipe from starting up smoothly, often leading to erratic temperature fluctuations.
Loss of Isothermal Performance
A properly functioning heat pipe is isothermal, meaning it maintains a nearly constant temperature across its length.
If the vacuum level is insufficient ($>10^{-6}$ Pa), the gas lock creates a temperature gradient, causing the condenser to run significantly cooler than the evaporator and defeating the purpose of the device.
Understanding the Trade-offs
Process Complexity vs. Reliability
Achieving a vacuum of $10^{-6}$ Pa requires sophisticated equipment, such as turbomolecular pumps or diffusion pumps, adding time and cost to the manufacturing process.
The Risk of shortcuts
Attempting to seal the pipe at a lower vacuum level (e.g., rough vacuum) might save processing time, but it guarantees a degradation in performance.
There is no "middle ground" for sodium heat pipes; even trace amounts of NCGs can expand significantly at operating temperatures, disabling the heat pipe.
Making the Right Choice for Your Goal
To ensure your sodium heat pipe meets its performance specifications, apply the following guidelines:
- If your primary focus is Maximum Heat Transfer: You must verify the vacuum system reaches at least $10^{-6}$ Pa to ensure zero thermal resistance at the condenser.
- If your primary focus is Reliable Startup: You must eliminate all NCGs to prevent vapor flow disruption during the critical initial heating phase.
Strict adherence to high-vacuum protocols is the only way to guarantee the efficient, isothermal operation of a sodium heat pipe.
Summary Table:
| Factor | Requirement | Impact of Failure |
|---|---|---|
| Vacuum Level | $10^{-6}$ Pa | Incomplete gas removal; system failure |
| Gas Type | Non-Condensable (NCGs) | Accumulate at cold end; create gas locks |
| Heat Transfer | Maximum Efficiency | Increased thermal resistance; insulation effect |
| Vapor Flow | Continuous & Smooth | Erratic temperature; startup disruptions |
| Isothermal State | Uniform Temperature | Large temperature gradients across the pipe |
Maximize Your Heat Pipe Performance with KINTEK
Don't let residual gases compromise your thermal management systems. Backed by expert R&D and precision manufacturing, KINTEK offers high-performance High-Vacuum, CVD, and Laboratory High-Temperature Furnace systems tailored for critical sealing processes. Whether you need customizable solutions for sodium heat pipes or advanced material research, our engineering team provides the reliability you need for a $10^{-6}$ Pa environment. Contact KINTEK today to discuss your unique needs!
Visual Guide
References
- Shuaijie Sha, Junjie Wang. Experimental and numerical simulation study of sodium heat pipe with large aspect ratio. DOI: 10.2298/tsci231030059s
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- 304 316 Stainless Steel High Vacuum Ball Stop Valve for Vacuum Systems
- Stainless Steel Quick Release Vacuum Chain Three Section Clamp
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
People Also Ask
- What is the role of an optical pyrometer in diffusion bonding? Ensure Precision in High-Temperature Simulations
- How does an in-situ reaction chamber in HTXRD facilitate BiFeO3 synthesis study? Mapping Real-Time Phase Evolution
- Why is the use of high-purity graphite crucibles essential? Protect TiC-High Manganese Steel During Sintering
- What precautions should be taken when using the alumina furnace tube for the first time? Ensure Safe Initial Use with Proper Conditioning
- Why is a MgO crucible preferred for VCD? Achieve 3ppm Purity in High-Temperature Metallurgy
- What is the primary function of the vacuum pump system in the magnesium powder evaporation process? Ensure High Purity & Efficiency
- Why are laboratory hydraulic presses critical for FMDS pelletization? Boost Strength Without Heat
- How do multi-bore high-purity alumina tubes stabilize CV tests? Enhance Data Accuracy with KINTEK Solutions



















