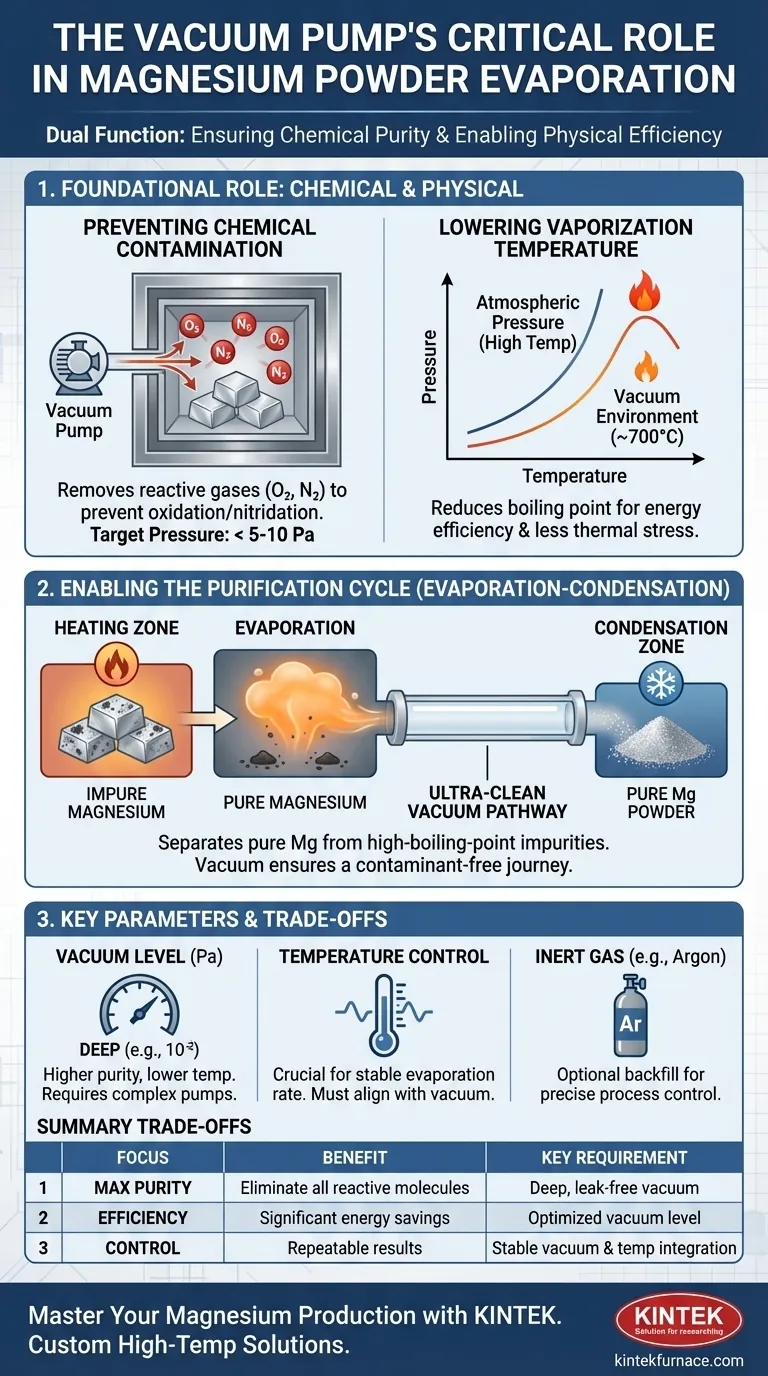
At its core, the vacuum pump system in magnesium powder production serves two equally critical functions: it prevents the chemical contamination of the highly reactive magnesium and fundamentally alters the physics of the process to enable efficient evaporation at lower, more manageable temperatures. By removing reactive gases like oxygen and creating a low-pressure environment, the vacuum pump is the key to producing high-purity magnesium powder effectively.
The vacuum system is not merely for cleaning the furnace. It's an active process-enabler that manipulates physical properties to make the high-purity evaporation and condensation of magnesium metal both possible and economically viable.

The Foundational Role of a High-Vacuum Environment
To understand the evaporation process, we must first appreciate the two distinct problems that the vacuum system solves simultaneously. One is a chemical problem (reactivity), and the other is a physical one (energy requirements).
Function 1: Preventing Chemical Contamination
Magnesium is highly reactive, especially at the elevated temperatures required for evaporation.
It readily combines with residual gases in the air. The primary contaminants are oxygen, which forms magnesium oxide (MgO), and nitrogen, which forms magnesium nitride (Mg₃N₂).
The vacuum pump's first job is to evacuate the process chamber to a pressure below 5-10 Pa. This physically removes the vast majority of these reactive gas molecules, creating an ultra-clean environment that prevents the magnesium from oxidizing or nitriding as it is heated.
Function 2: Lowering the Vaporization Temperature
The temperature at which a liquid boils is directly dependent on the pressure above it. Think of boiling water at high altitude—it boils at a lower temperature because the air pressure is lower.
The vacuum pump creates an extreme "high-altitude" environment inside the furnace. By reducing the pressure dramatically, it significantly lowers the boiling point of magnesium.
This allows the metal to vaporize efficiently at a much lower temperature (e.g., around 700°C) than it would at atmospheric pressure. This makes the entire process more energy-efficient and places less thermal stress on the equipment.
How Vacuum Enables the Purification Process
With contamination prevented and the vaporization temperature lowered, the vacuum system becomes the engine for the actual purification cycle.
The Evaporation-Condensation Mechanism
The process is designed to separate pure magnesium from less volatile impurities, such as other metals in an alloy or non-metallic slag.
By combining the low-pressure vacuum with precise heating, the system brings the material to a temperature where magnesium readily vaporizes, but the high-boiling-point impurities remain behind as a solid or liquid.
This pure magnesium vapor then travels through the vacuum chamber to a cooler surface, where it condenses back into a solid, forming the desired high-purity magnesium powder.
Creating an Ultra-Clean Pathway
The vacuum's role doesn't end once evaporation begins. The low-pressure environment ensures that the magnesium vapor can travel from the heating zone to the condensation zone without colliding with and reacting with air molecules.
This maintains the purity of the magnesium throughout its entire journey from raw material to final powder.
Understanding the Trade-offs and Key Parameters
A vacuum system is not a simple on/off switch. Its performance and integration with other systems are crucial for a successful outcome.
The Importance of Vacuum Level
The "deepness" of the vacuum—measured in Pascals (Pa) or mmHg—is a critical parameter. A deeper vacuum (lower pressure, like 10⁻² Pa) allows for even lower evaporation temperatures.
However, achieving and maintaining a deeper vacuum requires more powerful, complex, and expensive pumping systems. The optimal level is a trade-off between energy savings and equipment cost.
Interaction with Temperature Control
Vacuum and temperature are intrinsically linked. A stable vacuum is necessary for a predictable evaporation rate at a given temperature.
Any fluctuation in pressure will change the boiling point, making the process difficult to control. Therefore, a high-performance vacuum system must work in perfect harmony with a precise temperature control system.
The Role of Inert Gas
In some processes, after the initial evacuation, the chamber is backfilled with a high-purity inert gas like argon.
This is done to help control the rate of evaporation and condensation more precisely. The vacuum pump is still essential for the initial cleaning, but the inert gas provides an additional layer of process control.
Making the Right Choice for Your Goal
The specific focus for your vacuum system depends entirely on your end goal.
- If your primary focus is maximum purity: A deep, clean, and leak-free vacuum is non-negotiable to eliminate virtually all reactive molecules before heating begins.
- If your primary focus is process efficiency: The vacuum level must be optimized to lower the vaporization temperature just enough to achieve significant energy savings without requiring a prohibitively expensive pump system.
- If your primary focus is process control and repeatability: The key is the stability of the vacuum and its seamless integration with the furnace's temperature controls to manage evaporation and condensation rates precisely.
Ultimately, mastering the vacuum environment is essential for unlocking the controlled production of high-quality magnesium powder.
Summary Table:
| Function | Key Benefit | Key Parameter |
|---|---|---|
| Prevent Chemical Contamination | Eliminates oxidation/nitridation by removing reactive gases (O₂, N₂) | Pressure < 5-10 Pa |
| Lower Vaporization Temperature | Reduces energy use and thermal stress by lowering boiling point | Lower pressure = lower temperature |
| Enable Purification Cycle | Separates pure Mg vapor from high-boiling-point impurities | Stable vacuum & temperature control |
| Ensure Ultra-Clean Pathway | Allows vapor to travel and condense without reacting | Deep, stable vacuum level (e.g., 10⁻² Pa) |
Ready to Master Your Magnesium Powder Production?
Producing high-purity, consistent magnesium powder requires precise control over the vacuum environment. The right furnace and vacuum system are critical to preventing contamination, improving energy efficiency, and ensuring repeatable results.
KINTEK is your partner in high-temperature processing. Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems, including lab high-temp furnaces, all customizable for your unique needs.
Let us help you optimize your process. Contact our experts today to discuss your application and discover the ideal solution for your goals.
Visual Guide
Related Products
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Ultra Vacuum Electrode Feedthrough Connector Flange Power Lead for High Precision Applications
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- CF KF Flange Vacuum Electrode Feedthrough Lead Sealing Assembly for Vacuum Systems
People Also Ask
- What industrial and research applications are tube furnaces used for? Unlock Precise Thermal Processing Solutions
- What role do tube furnaces play in semiconductor and battery production? Unlock Precision in High-Temp Processing
- Why is a high-precision vacuum tube furnace essential for CVD graphene? Master Growth Control & Purity
- What materials are used for the tubes in a High Temperature Tube Furnace? Choose the Right Tube for Your Lab
- What is the function of high-vacuum encapsulated quartz tubes for Ce2(Fe, Co)17? Ensure Phase Purity and Stability



















