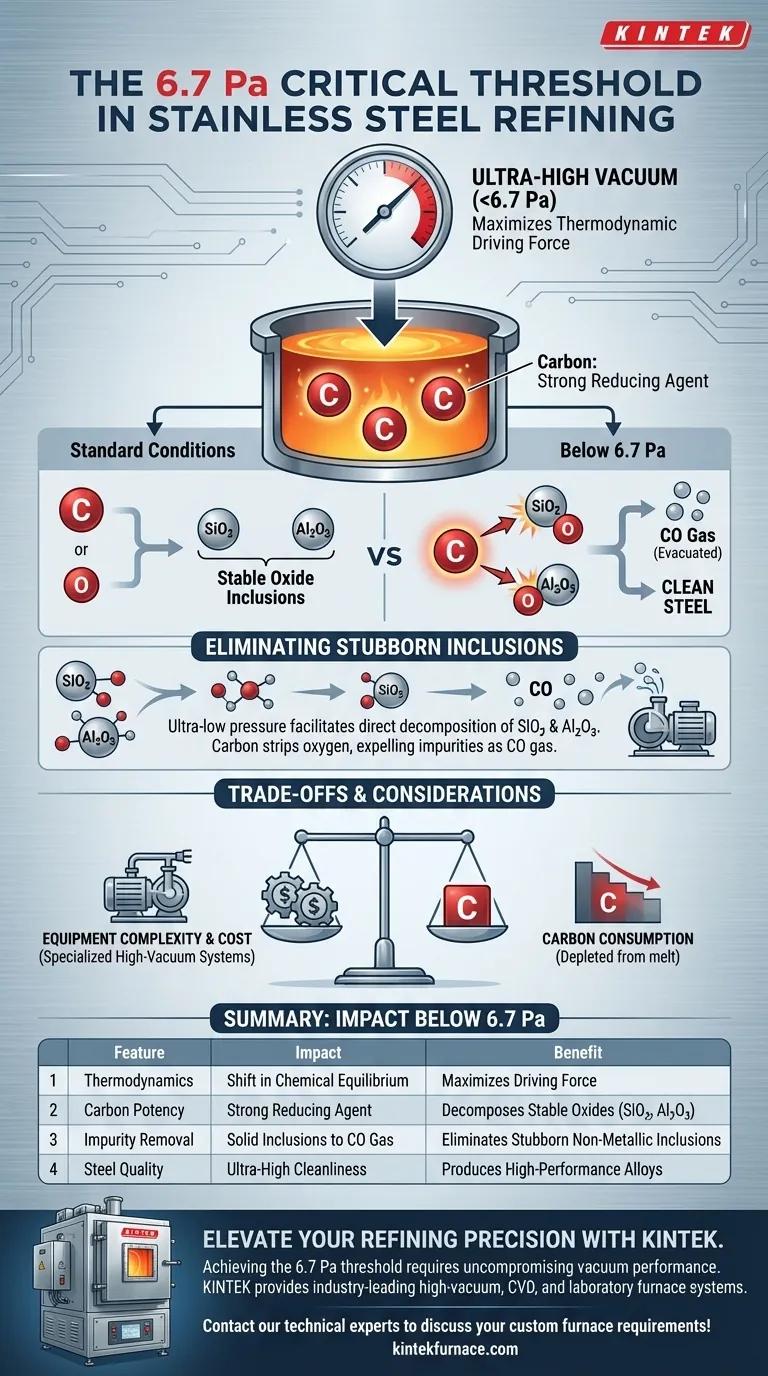
The specific threshold of 6.7 Pa is critical for maximizing the thermodynamic driving force of the refining process. Maintaining a high vacuum below this limit drastically increases the potential for the carbon-oxygen reaction within molten steel. This environment transforms carbon into a potent reducing agent capable of breaking down stubborn impurities that higher-pressure methods cannot remove.
By lowering the system pressure below 6.7 Pa, you shift the thermodynamic equilibrium to favor the decomposition of stable oxide inclusions. This allows carbon to strip oxygen from impurities like silicon dioxide and aluminum oxide, expelling them as gas to achieve ultra-high cleanliness.

The Thermodynamics of High Vacuum
Shifting the Chemical Equilibrium
According to thermodynamic equilibrium theory, pressure is not just a physical force; it is a control knob for chemical potency. When you reduce the environment to ultra-low partial pressures, you fundamentally alter how elements within the melt interact.
Unleashing Carbon’s Reducing Power
Under standard conditions, carbon is simply an alloying element. However, below 6.7 Pa, carbon gains strong reducing power. This shift allows it to aggressively seek out and bond with oxygen atoms that are otherwise locked in stable compounds.
Eliminating Stable Inclusions
Targeting Hard-to-Remove Oxides
In standard refining, stable oxide inclusions such as silicon dioxide (SiO2) and aluminum oxide (Al2O3) are notoriously difficult to eliminate. They are chemically stable and tend to remain suspended in the steel, compromising its quality.
The Gas Phase Removal Mechanism
The ultra-high vacuum facilitates the direct decomposition of these stubborn oxides. The empowered carbon reacts with the oxygen inside the SiO2 and Al2O3, converting the solid impurity into carbon monoxide (CO) gas. This gas is then easily evacuated by the pumping system, leaving the steel significantly cleaner.
Understanding the Trade-offs
Equipment Complexity
Achieving a vacuum level of 6.7 Pa is not a trivial task; it requires a specialized high-vacuum pumping system. This increases the operational complexity and equipment cost compared to standard vacuum degassing, which operates at higher pressures.
Carbon Consumption
Because the mechanism relies on carbon reacting with oxygen to form CO, the process naturally depletes carbon from the melt. You must carefully calculate the initial carbon content to ensure the final product meets the necessary chemical specifications after the refining reaction is complete.
Making the Right Choice for Your Refining Goals
Leveraging this pressure threshold is essential for projects demanding the highest purity levels.
- If your primary focus is ultra-high cleanliness: Ensure your pumping infrastructure is robust enough to sustain pressures below 6.7 Pa to activate the decomposition of Al2O3 and SiO2.
- If your primary focus is reaction kinetics: Monitor the vacuum stability closely, as fluctuations above 6.7 Pa will immediately reduce the driving force and halt the removal of stable oxides.
Mastering this vacuum threshold is the key to transitioning from standard stainless steel to ultra-clean, high-performance alloys.
Summary Table:
| Feature | Impact Below 6.7 Pa | Benefit |
|---|---|---|
| Thermodynamics | Shift in chemical equilibrium | Maximizes driving force for refining |
| Carbon Potency | Becomes a strong reducing agent | Decomposes stable oxides like Al2O3 & SiO2 |
| Impurity Removal | Solid inclusions converted to CO gas | Eliminates stubborn non-metallic inclusions |
| Steel Quality | Ultra-high cleanliness levels | Produces high-performance, clean alloys |
Elevate Your Refining Precision with KINTEK
Achieving the critical 6.7 Pa threshold requires uncompromising vacuum performance. KINTEK provides industry-leading high-vacuum, CVD, and customizable laboratory furnace systems designed to meet the rigorous demands of advanced metallurgical research.
Backed by expert R&D and precision manufacturing, our equipment ensures you maintain the stable, ultra-low partial pressures necessary to unleash carbon's reducing power and eliminate stable oxide inclusions. Whether you are developing high-performance alloys or refining stainless steel, KINTEK delivers the reliability you need to master your thermodynamic goals.
Ready to optimize your high-vacuum refining process? Contact our technical experts today to discuss your custom furnace requirements!
Visual Guide
References
- Shunsuke Narita, Yoshinori Sumi. Effect of deoxidizing elements on inclusions in vacuum refining of stainless steel. DOI: 10.1088/1757-899x/1329/1/012005
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
People Also Ask
- What role does a high-temperature vacuum furnace play in the synthesis of LaTiOC/NdTiOC? Master Heteroanionic Materials
- What role do vacuum furnaces play in modern industrial production? Essential for High-Purity, High-Performance Manufacturing
- What are the advantages of using a vacuum dryer for cerium oxide nanoparticles? Preserve Integrity & Prevent Oxidation
- What is the purpose of using a vacuum oven for h-NCM(OH)2? Optimize Your Cathode Material Research
- Why is oxidation a concern when heating metals, and how does a vacuum furnace address this? Ensure Purity and Performance
- What is the necessity of vacuum drying equipment for ball-milled powders? Ensure Purity & Density in Ceramics
- What are the key components of a vacuum annealing furnace? Master the Core for Superior Material Processing
- What are the key advantages of vacuum furnaces? Achieve Superior Heat Treatment for Your Materials



















