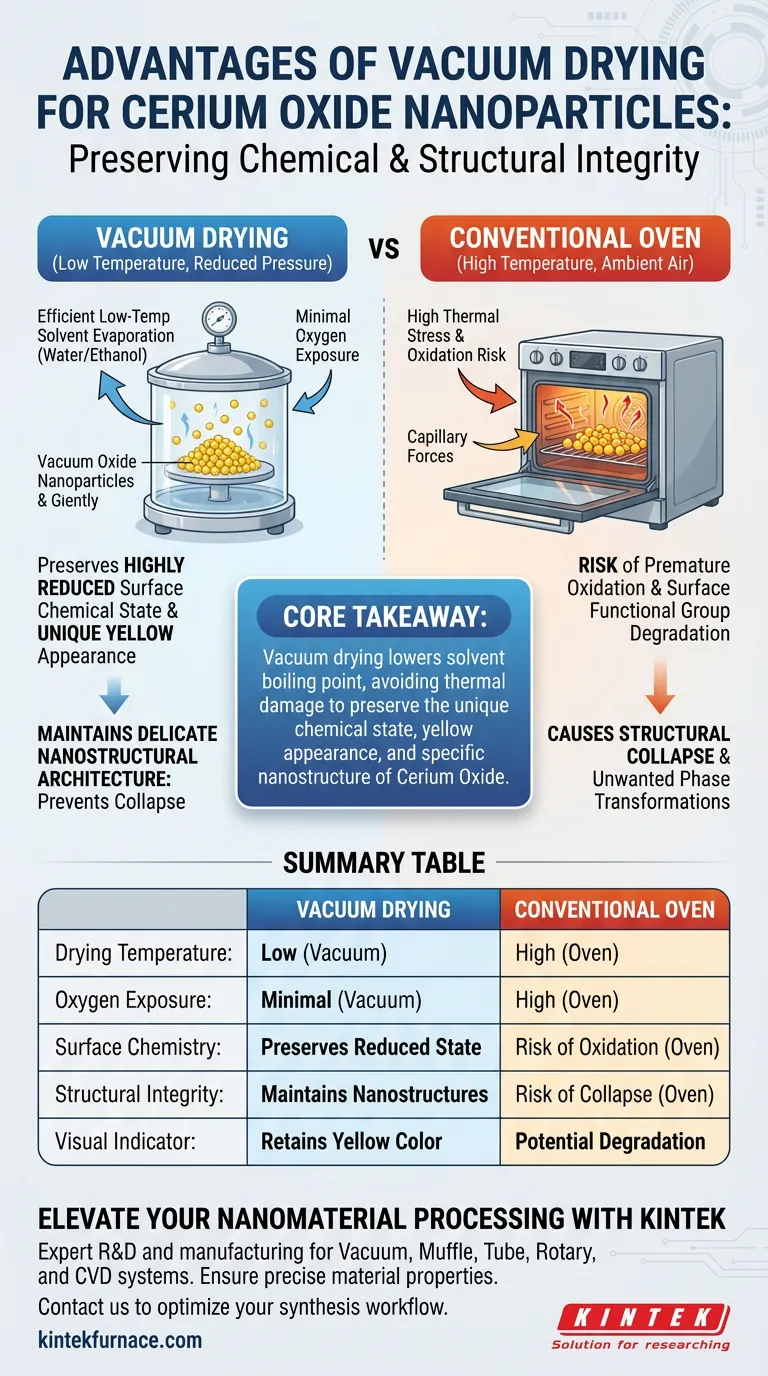
The primary advantage of using a vacuum dryer for cerium oxide nanoparticles is the preservation of chemical and structural integrity. By operating under reduced pressure, this method allows for the efficient evaporation of solvents like water and ethanol at significantly lower temperatures, thereby avoiding the thermal damage associated with conventional ovens.
Core Takeaway Conventional high-temperature drying risks oxidizing surface functional groups and collapsing delicate nanostructures. Vacuum drying circumvents these issues by lowering the solvent boiling point, effectively preserving the cerium oxide's highly reduced surface chemical state, unique yellow appearance, and specific nanostructural architecture.

Preserving Chemical State and Surface Functionality
Preventing Premature Oxidation
In a conventional oven, high temperatures combined with ambient air exposure can lead to rapid oxidation. Vacuum drying creates an environment that minimizes oxygen exposure.
For superhydrophobic cerium oxide, this is critical. It prevents the premature oxidation of surface functional groups, ensuring the material retains its intended chemical properties.
Maintaining the Reduced Surface State
The effectiveness of cerium oxide is often tied to its surface chemistry. Vacuum drying maintains the material in a highly reduced surface chemical state.
This preservation is visually evident; the process ensures the samples retain their unique yellow appearance, which indicates that the desired chemical composition has not been compromised by heat or oxygen.
Maintaining Nanostructural Architecture
Preventing Structural Collapse
Nanomaterials are structurally delicate. The high heat of conventional ovens can cause the collapse of nanostructures, destroying the specific morphology required for the material's application.
Vacuum drying mitigates this risk by removing solvents gently. This ensures the physical framework of the nanoparticles remains intact, rather than densifying or degrading.
Efficient Low-Temperature Solvent Removal
The fundamental mechanism driving these benefits is the relationship between pressure and boiling points. A vacuum dryer reduces the internal pressure, allowing residual solvents (such as ethanol and water) to evaporate rapidly at low temperatures.
This allows the material to dry thoroughly without being subjected to the thermal stress that typically triggers physical degradation.
Understanding the Risks of Conventional Methods
The Pitfalls of High-Temperature Air Drying
While conventional ovens are common, they introduce "capillary forces" and "thermal stresses" during liquid-phase evaporation (as noted in broader contexts of nanomaterial drying).
For cerium oxide specifically, reliance on a standard oven increases the probability of unwanted phase transformations or surface degradation. If the priority is high-performance material synthesis, the convenience of a standard oven is outweighed by the risk of altering the material's fundamental characteristics.
Making the Right Choice for Your Goal
To ensure the highest quality synthesis of cerium oxide nanoparticles, align your drying method with your specific material requirements:
- If your primary focus is Surface Chemistry: Choose vacuum drying to prevent oxidation and maintain the highly reduced surface state and functional groups essential for reactivity.
- If your primary focus is Structural Integrity: Utilize vacuum drying to avoid the collapse of nanostructures that frequently occurs under high-temperature thermal stress.
Ultimately, vacuum drying is not merely a method of moisture removal, but a critical processing step that defines the final quality and utility of the nanomaterial.
Summary Table:
| Feature | Vacuum Drying | Conventional Oven Drying |
|---|---|---|
| Drying Temperature | Low (reduces solvent boiling point) | High (requires high thermal energy) |
| Oxygen Exposure | Minimal (vacuum environment) | High (ambient air) |
| Surface Chemistry | Preserves reduced chemical state | Risk of premature oxidation |
| Structural Integrity | Maintains delicate nanostructures | Risk of structural collapse |
| Visual Indicator | Retains unique yellow appearance | Potential color change/degradation |
Elevate Your Nanomaterial Processing with KINTEK
Don't compromise the integrity of your cerium oxide nanoparticles with inferior drying methods. KINTEK provides industry-leading thermal solutions designed for the most delicate laboratory requirements. Backed by expert R&D and manufacturing, we offer a comprehensive range of customizable Vacuum, Muffle, Tube, Rotary, and CVD systems to ensure your materials retain their precise chemical and structural properties.
Ready to optimize your synthesis workflow? Contact us today to find the perfect drying solution for your unique needs.
Visual Guide
References
- Kaline Nunes dos Santos, Fabiano Bernardi. Engineering Pt–CeO<sub>2</sub>interfaces for reverse water-gas shift (RWGS) reaction. DOI: 10.1039/d4lf00064a
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Sintering and Brazing Furnace
- Vacuum Hot Press Furnace Machine for Lamination and Heating
People Also Ask
- What are the characteristics of vacuum heat treatment? Unlock Superior Material Performance and Control
- What are the benefits of vacuum sintering? Achieve Superior Material Properties and Purity
- What are the cooling methods for vacuum sintering furnaces? Optimize Your Material Properties
- What is the purpose of an annealing furnace in the mechanical industry? Boost Metal Performance and Efficiency
- What are the process advantages of RTT vs. vacuum annealing for nickel-silicon? Achieve precise sub-micron control
- Why is temperature stability important in vacuum furnace operations? Ensure Precise Heat Treatment for Superior Materials
- How does the heating mechanism of an SPS furnace benefit CNT copper composites? Preserve Nanostructures with Rapid Heat
- What is the function of a high-temperature debinding and sintering furnace in BMD? Your Path to Solid Metal Parts



















