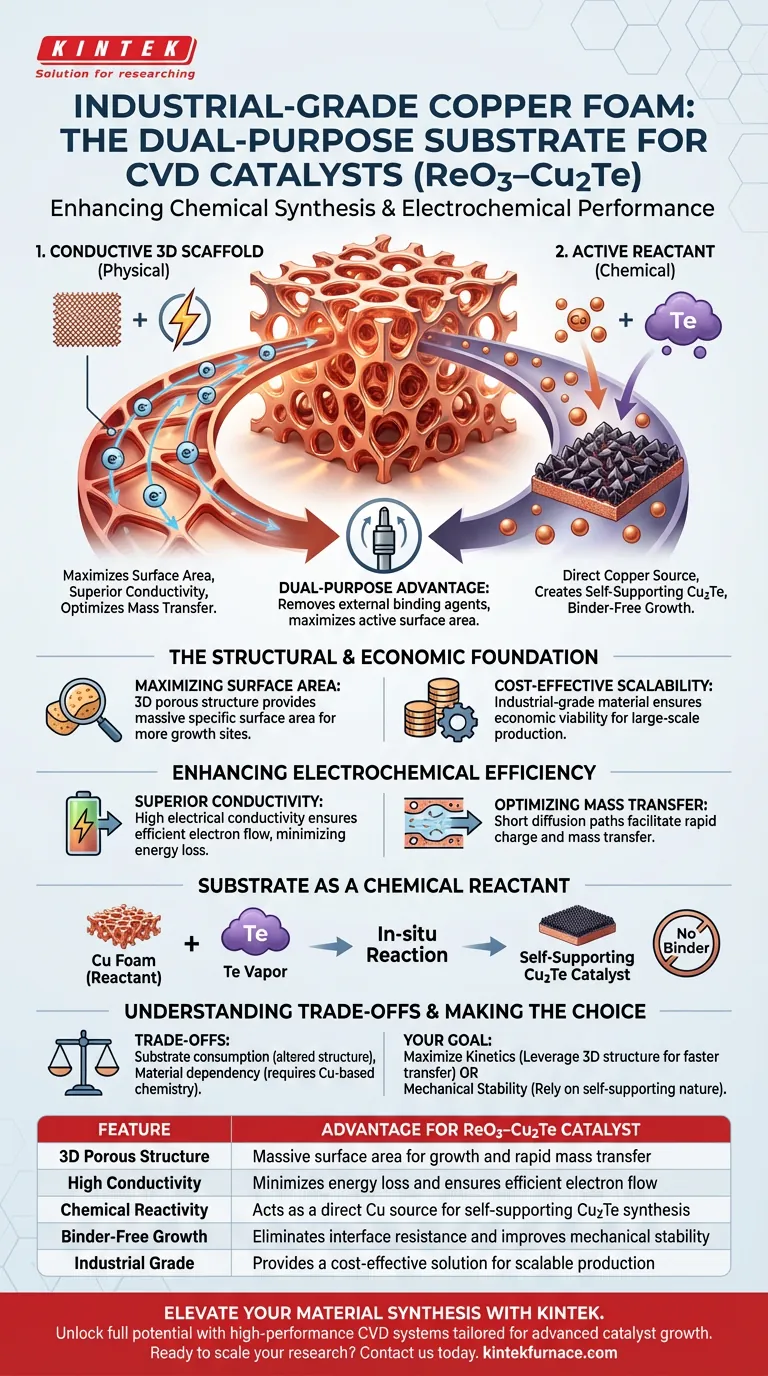
Industrial-grade copper foam acts as a dual-purpose substrate that significantly enhances both the chemical synthesis and the electrochemical performance of ReO3–Cu2Te catalysts. It serves not only as a conductive 3D scaffold with high surface area for material growth but also as an active reactant, directly supplying copper to form the self-supporting catalyst structure during Chemical Vapor Deposition (CVD).
By functioning simultaneously as a physical current collector and a chemical precursor, copper foam eliminates the need for external binding agents while maximizing the active surface area available for catalytic reactions.
The Structural and Economic Foundation
Maximizing Surface Area
The defining physical characteristic of copper foam is its three-dimensional porous structure. This architecture provides a massive specific surface area compared to flat substrates, offering significantly more sites for catalyst growth.
Cost-Effective Scalability
The use of industrial-grade material ensures the process remains economically viable. This low-cost availability is essential for scaling up production without incurring prohibitive material expenses.
Enhancing Electrochemical Efficiency
Superior Conductivity
Copper is utilized for its intrinsic high electrical conductivity. This property ensures efficient electron flow throughout the electrode, minimizing energy loss during operation.
Optimizing Mass Transfer
The porous nature of the foam creates short diffusion paths. This facilitates rapid charge and mass transfer, which is particularly critical for maintaining efficiency during the hydrogen evolution process.
The Substrate as a Chemical Reactant
Direct Precursor Reaction
Unlike inert substrates that merely hold a catalyst, copper foam actively participates in the CVD process. It acts as a direct copper source, reacting with tellurium vapor to synthesize the active material.
Creating Self-Supporting Structures
This in-situ reaction results in the formation of self-supporting copper telluride (Cu2Te). This eliminates the interface resistance often found in coated electrodes and enhances electron transfer efficiency between the active material and the current collector.
Understanding the Trade-offs
Substrate Consumption
Because the foam acts as a reactant, the substrate is inherently altered during the process. The reaction consumes part of the copper structure, requiring precise process control to preserve the mechanical framework.
Material Dependency
The benefits of this approach are tied strictly to the chemistry of the substrate. This method is only viable for applications where the formation of copper-based compounds (like copper telluride) is chemically desirable.
Making the Right Choice for Your Goal
To determine if this substrate alignment fits your specific engineering requirements, consider the following:
- If your primary focus is maximizing reaction kinetics: Leverage the 3D porous structure to shorten diffusion paths and increase the density of active sites for faster mass transfer.
- If your primary focus is mechanical stability: Rely on the self-supporting nature of the in-situ growth to create a robust connection between the catalyst and current collector without binders.
Ultimately, selecting copper foam transforms the substrate from a passive component into an active, performance-enhancing element of the catalyst system.
Summary Table:
| Feature | Advantage for ReO3–Cu2Te Catalyst |
|---|---|
| 3D Porous Structure | Massive surface area for growth and rapid mass transfer |
| High Conductivity | Minimizes energy loss and ensures efficient electron flow |
| Chemical Reactivity | Acts as a direct Cu source for self-supporting Cu2Te synthesis |
| Binder-Free Growth | Eliminates interface resistance and improves mechanical stability |
| Industrial Grade | Provides a cost-effective solution for scalable production |
Elevate Your Material Synthesis with KINTEK
Unlock the full potential of your CVD processes with high-performance equipment tailored for advanced catalyst growth. Backed by expert R&D and manufacturing, KINTEK offers state-of-the-art Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet your unique laboratory needs. Whether you are working with industrial copper foam or developing next-generation self-supporting structures, our systems ensure the precise thermal and chemical control required for success.
Ready to scale your research? Contact us today to consult with our specialists and find the perfect high-temperature solution for your lab.
Visual Guide
References
- Aruna Vijayan, N. Sandhyarani. Efficient and sustainable hydrogen evolution reaction: enhanced photoelectrochemical performance of ReO<sub>3</sub>-incorporated Cu<sub>2</sub>Te catalysts. DOI: 10.1039/d4ya00023d
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- Cylindrical Resonator MPCVD Machine System for Lab Diamond Growth
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Split Chamber CVD Tube Furnace with Vacuum Station CVD Machine
People Also Ask
- What is the operating principle of a Quartz Crystal Thickness Monitor? Achieve Precise ZTO Thin Film Control
- What are the main challenges in CVD technology? Overcome Key Hurdles for Better Thin-Film Deposition
- How does high vacuum thermal evaporation equipment contribute to Cu2SnS3 (CTS) PVD? High-Purity Film Solutions
- What is the function of PVD Vacuum Arc Evaporation for CrAlSiN coatings? Boost Tool Durability with High-Energy Plasma
- What are some applications of CVD? Unlock Precision in Electronics, Aerospace, and Materials
- What are the key application fields of CVD tube furnaces? Unlock Precision in Thin-Film Synthesis
- Is PVD the same as CVD? Understanding the Physical vs. Chemical Deposition Difference
- What are some biomedical applications of CVD? Enhance Medical Device Safety and Longevity
















