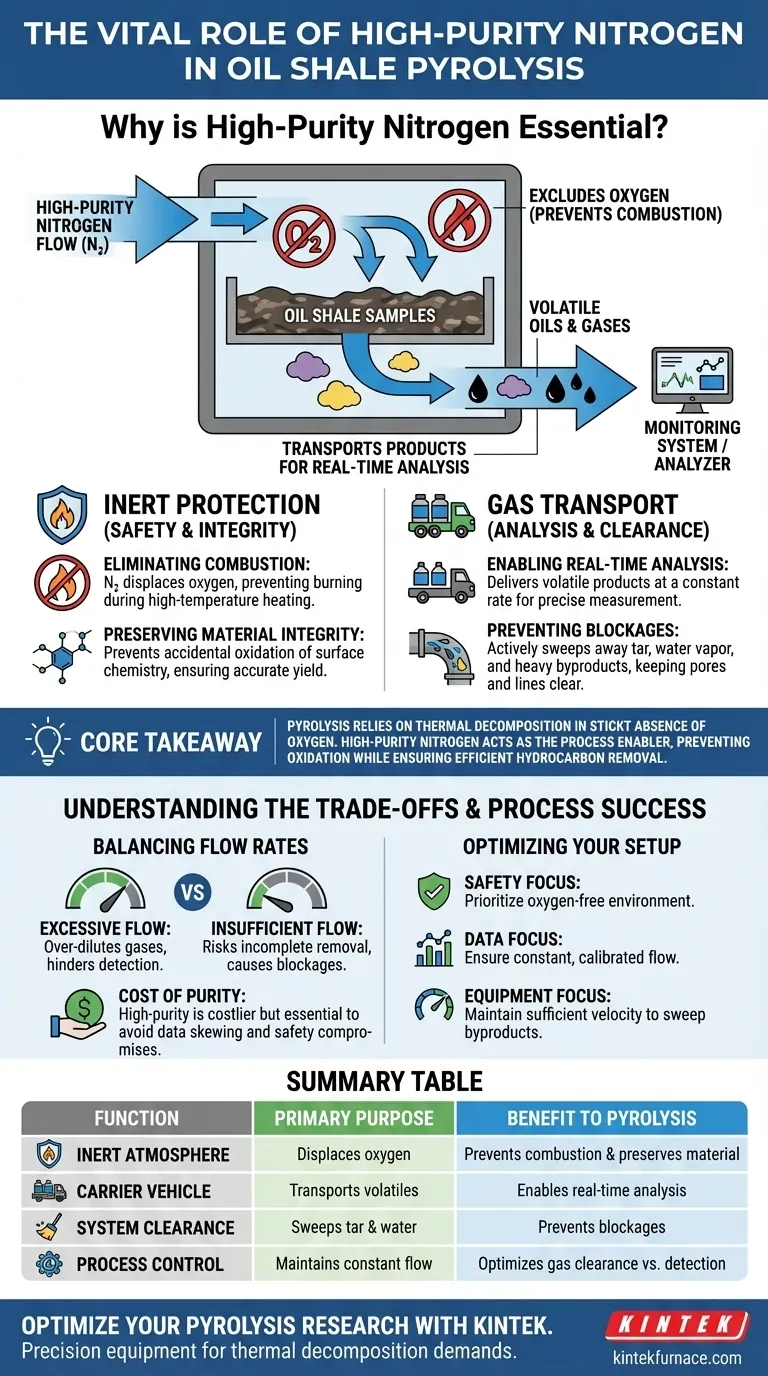
High-purity nitrogen serves as the essential stabilizer in oil shale pyrolysis, functioning primarily to exclude oxygen and transport volatile products. By establishing an inert atmosphere, it prevents the oil shale from combusting at high processing temperatures, ensuring that thermal decomposition occurs rather than burning. Simultaneously, the nitrogen flow carries generated oil and gases to monitoring systems for accurate, real-time analysis.
Core Takeaway: Pyrolysis relies on thermal decomposition in the strict absence of oxygen. High-purity nitrogen acts as the process enabler, preventing unwanted oxidation while ensuring the efficient, unobstructed removal of hydrocarbons and byproducts for analysis.

The Role of Inert Protection
Eliminating the Risk of Combustion
Pyrolysis requires heating oil shale to extreme temperatures to break down kerogen into oil and gas.
If oxygen were present during this heating phase, the material would simply burn (oxidize) rather than decompose.
High-purity nitrogen displaces oxygen in the heating chamber, creating the inert environment necessary for safe thermal decomposition.
Preserving Material Integrity
Beyond preventing fire, nitrogen protects the chemical structure of the carbon material.
Accidental oxidation can alter the surface chemistry of the shale, ruining the sample before valuable hydrocarbons can be extracted.
A continuous flow of inert gas maintains the activity of the reaction interface, ensuring the process yields the intended chemical products.
The Functions of Gas Transport
Enabling Real-Time Analysis
The nitrogen acts as a vehicle, physically carrying the evolved gases and oils out of the reactor.
To analyze the output accurately, these volatile products must be delivered to monitoring systems at a constant, controlled rate.
This steady flow allows for precise, real-time measurement of gas concentrations as they are generated.
Preventing System Blockages
During pyrolysis, the shale releases tar, water vapor, and various volatile decomposition products.
If these heavy byproducts are allowed to stagnate, they can settle and block the internal pores of the material or the reactor lines.
Nitrogen flow at specific rates (e.g., 150 cm³/min) actively sweeps these byproducts away, preventing pore blockage and ensuring the reactor remains operational.
Understanding the Trade-offs
Balancing Flow Rates
While nitrogen flow is critical, the rate of flow introduces a trade-off between clearance and concentration.
Excessively high flow rates can over-dilute the product gases, making detection by monitoring equipment more difficult or less accurate.
Conversely, insufficient flow rates risk incomplete removal of tar and water, leading to the pore blockages mentioned above.
The Cost of Purity
Using high-purity nitrogen is more expensive than using standard industrial air or lower-grade inert gases.
However, using low-purity nitrogen introduces trace oxygen or moisture, which can skew experimental data and compromise the safety of the pyrolysis chamber.
Ensuring Process Success
To optimize your pyrolysis setup, consider your specific operational goals:
- If your primary focus is Safety and Sample Integrity: Prioritize maintaining a strictly oxygen-free environment to prevent combustion and surface oxidation.
- If your primary focus is Data Accuracy: Ensure the nitrogen flow rate is constant and calibrated to transport products to the analyzer without over-diluting them.
- If your primary focus is Equipment Longevity: maintain sufficient flow velocity to effectively sweep tar and water vapor from the reactor to prevent clogging.
High-purity nitrogen is not just a passive medium; it is an active component that secures the safety, chemistry, and measurability of the entire pyrolysis operation.
Summary Table:
| Function | Primary Purpose | Benefit to Pyrolysis |
|---|---|---|
| Inert Atmosphere | Displaces oxygen in the chamber | Prevents combustion and preserves material integrity |
| Carrier Vehicle | Transports volatile oils and gases | Enables real-time monitoring and accurate analysis |
| System Clearance | Sweeps away tar and water vapor | Prevents pore blockage and reactor line clogging |
| Process Control | Maintains constant flow rates | Optimizes the balance between gas clearance and detection accuracy |
Optimize Your Pyrolysis Research with KINTEK
Precision in oil shale pyrolysis starts with the right environment. KINTEK provides high-performance lab equipment designed to meet the rigorous demands of thermal decomposition research. Backed by expert R&D and manufacturing, we offer customizable Muffle, Tube, Rotary, Vacuum, and CVD systems tailored to your unique high-temperature needs. Whether you require precise gas flow control or robust furnace durability, our systems ensure your experiments remain safe, accurate, and efficient.
Ready to elevate your lab's thermal processing capabilities? Contact us today to find your custom solution!
Visual Guide
References
- Yuping Yuan, Zhiyong Chang. Deep Learning Framework for Oil Shale Pyrolysis State Recognition Using Bionic Electronic Nose. DOI: 10.1007/s44196-025-00913-5
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant Rotating Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What is a tube furnace and what is its primary use? Achieve Precision High-Temp Processing for Your Lab
- Why must high-purity argon gas be introduced during PTL sintering? Protect Titanium Integrity in Tube Furnaces
- How does a tube furnace control the phase structure of copper-based chalcogenides? Master Precise Thermal Management
- What materials are used for the chamber and insulation in three-zone split tube furnaces? Optimize Your High-Temp Processes
- How does a tube furnace facilitate the carbonization of ZIFs while preventing oxidation? Expert Insights
- Why is a tube furnace required for the calcination of TiO2 in an H2/Ar mixed atmosphere? Engineering TiO2-X Defects
- How does a high-temperature tube furnace facilitate the sintering process of modified graphite felt? Precision Control
- How does a tube furnace contribute to the synthesis of electrocatalysts from hydrochar? Precision Thermal Engineering



















