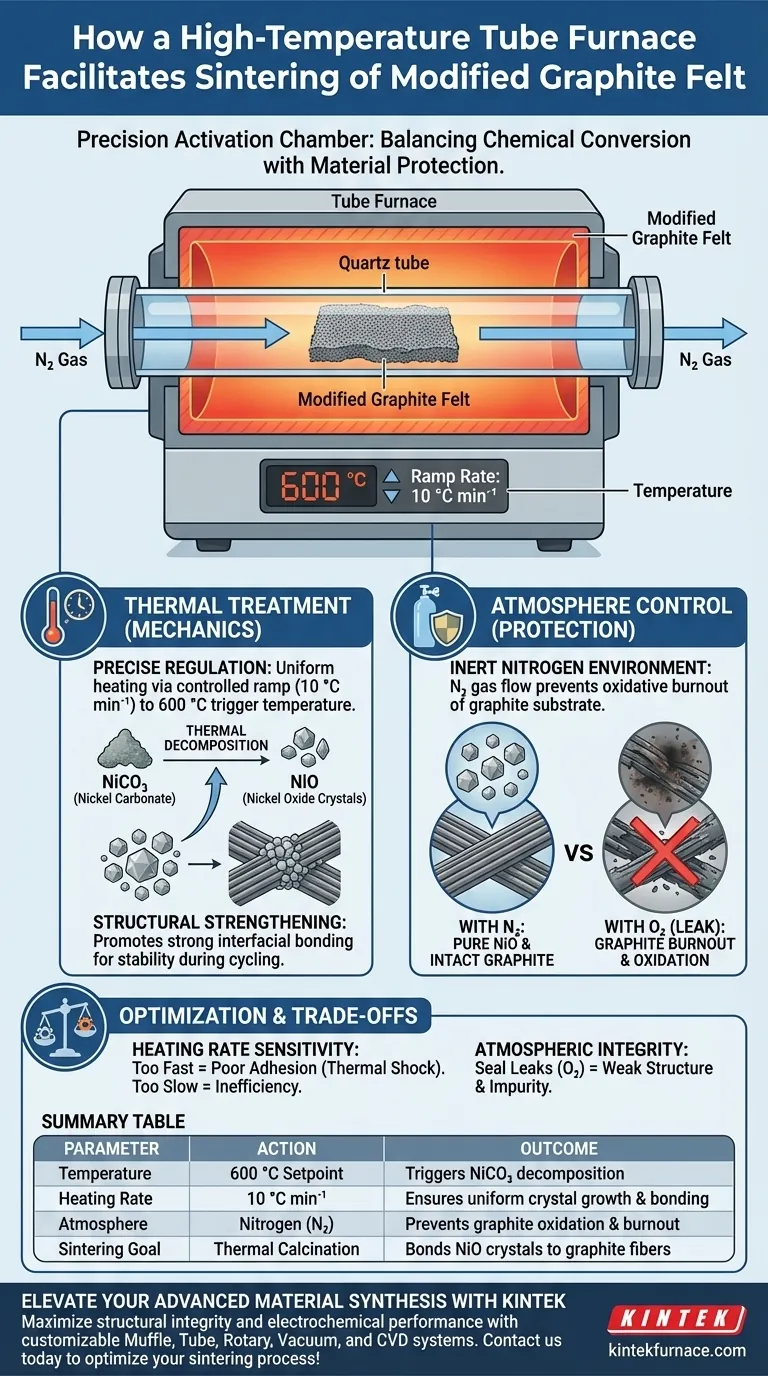
A high-temperature tube furnace facilitates the sintering process of modified graphite felt by providing a strictly controlled thermal and atmospheric environment. Specifically, it heats the material to 600 °C at a controlled rate (e.g., 10 °C min⁻¹) under nitrogen protection to thermally decompose nickel carbonate into stable nickel oxide crystals without damaging the graphite substrate.
Core Takeaway The tube furnace serves as a precision activation chamber that balances chemical conversion with material protection. It facilitates the transformation of precursors into active crystals while strengthening the physical bond between these crystals and the graphite fibers, which is essential for structural stability during battery cycling.

The Mechanics of Thermal Treatment
Precise Temperature Regulation
The primary function of the furnace is to execute a specific thermal profile. By ramping the temperature at a controlled rate, such as 10 °C min⁻¹, the furnace ensures uniform heating across the graphite felt.
Reaching the target temperature of 600 °C is critical. This specific thermal energy is required to trigger the decomposition of the precursor material (nickel carbonate) effectively.
Chemical Transformation
Inside the heated zone, the furnace drives a chemical reaction known as thermal decomposition.
The heat converts the nickel carbonate applied to the felt into nickel oxide crystals. This conversion is the core "sintering" or calcination step that activates the material for its intended electrochemical use.
Structural Strengthening
Beyond simple chemical conversion, the thermal treatment changes the physical mechanics of the material.
The high temperature promotes a strong interfacial bond between the newly formed nickel oxide crystals and the graphite fiber surface. This adhesion is vital; without it, the active material would detach during the rigorous flow battery cycling process.
The Role of Atmosphere Control
Preventing Material Degradation
Graphite felt is highly susceptible to oxidation at elevated temperatures. If processed in standard air at 600 °C, the graphite fibers would suffer oxidative burnout, destroying the electrode structure.
The tube furnace mitigates this by maintaining a sealed, inert atmosphere.
Inert Nitrogen Environment
To ensure the process is successful, the furnace fills the tube with nitrogen gas.
This creates an oxygen-free environment that serves two purposes: it protects the graphite from burning and ensures the nickel carbonate decomposes accurately into the target oxide without unwanted side reactions.
Understanding the Trade-offs
Sensitivity to Heating Rates
While rapid heating might seem efficient, deviating from the optimal rate (e.g., 10 °C min⁻¹) can be detrimental.
Excessive ramp rates can lead to thermal shock or uneven crystal formation, resulting in poor adhesion between the oxide and the graphite. Conversely, heating too slowly wastes energy and prolongs process time unnecessarily.
Atmospheric Integrity
The effectiveness of the sintering process is entirely dependent on the seal of the tube furnace.
Even minor leaks in the nitrogen supply can introduce oxygen. This compromises the purity of the nickel oxide and weakens the structural integrity of the graphite felt, leading to premature failure in application.
Making the Right Choice for Your Goal
To optimize the sintering of modified graphite felt, align your furnace settings with your specific performance metrics:
- If your primary focus is Structural Stability: Prioritize precise ramp rate control (10 °C min⁻¹) to ensure the nickel oxide bonds firmly to the graphite fibers for durability during cycling.
- If your primary focus is Chemical Purity: Focus on the integrity of the nitrogen atmosphere system to prevent side reactions and oxidative burnout of the graphite substrate.
Success depends on balancing thermal energy for conversion with atmospheric control for protection.
Summary Table:
| Process Parameter | Action | Outcome |
|---|---|---|
| Temperature | 600 °C Setpoint | Triggers nickel carbonate decomposition |
| Heating Rate | 10 °C min⁻¹ | Ensures uniform crystal growth & bonding |
| Atmosphere | Nitrogen (N2) | Prevents graphite oxidation & burnout |
| Sintering Goal | Thermal Calcination | Bonds NiO crystals to graphite fibers |
Elevate Your Advanced Material Synthesis with KINTEK
Maximize the structural integrity and electrochemical performance of your modified graphite felt with KINTEK’s precision thermal solutions. Backed by expert R&D and manufacturing, KINTEK offers customizable Muffle, Tube, Rotary, Vacuum, and CVD systems designed to meet the rigorous demands of battery research and industrial sintering.
Whether you require ultra-stable ramp rates or superior atmospheric integrity, our high-temperature furnaces provide the reliability your lab needs. Contact us today to optimize your sintering process!
Visual Guide
References
- Jingping Xie, Xiao‐min Wang. Performance Study of Nickel Oxide Graphite Felts as Electrode Materials for Ferrochromium Flow Batteries. DOI: 10.1002/open.202500405
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- What are the disadvantages of a tube furnace? Key Limitations for Industrial and Lab Use
- What are the limitations of horizontal tube furnaces? Manage Space, Temperature, and Handling Challenges
- Why is high-purity quartz tube vacuum sealing required for Ag2S1-xTex? Protect Your Semiconductor Synthesis
- What are the types of Tube Furnaces based on orientation? Horizontal vs. Vertical for Optimal Thermal Processing
- How is a high-precision laboratory balance installed in a tube furnace? Master Thermal Isolation for Precise Data
- Why is heat treatment in a tube furnace or muffle furnace required after synthesizing magnesium hydroxide nano-precursors via electrochemical methods? Unlock the Full Potential of Your MgO Nanomaterials
- What role does a high-temperature tube furnace play in Pt/MoS2 synthesis? Master Atomic-Level Defect Engineering
- Why is a High-Temperature Vacuum Tube Furnace required for the long-term homogenization of alloy ingots?



















