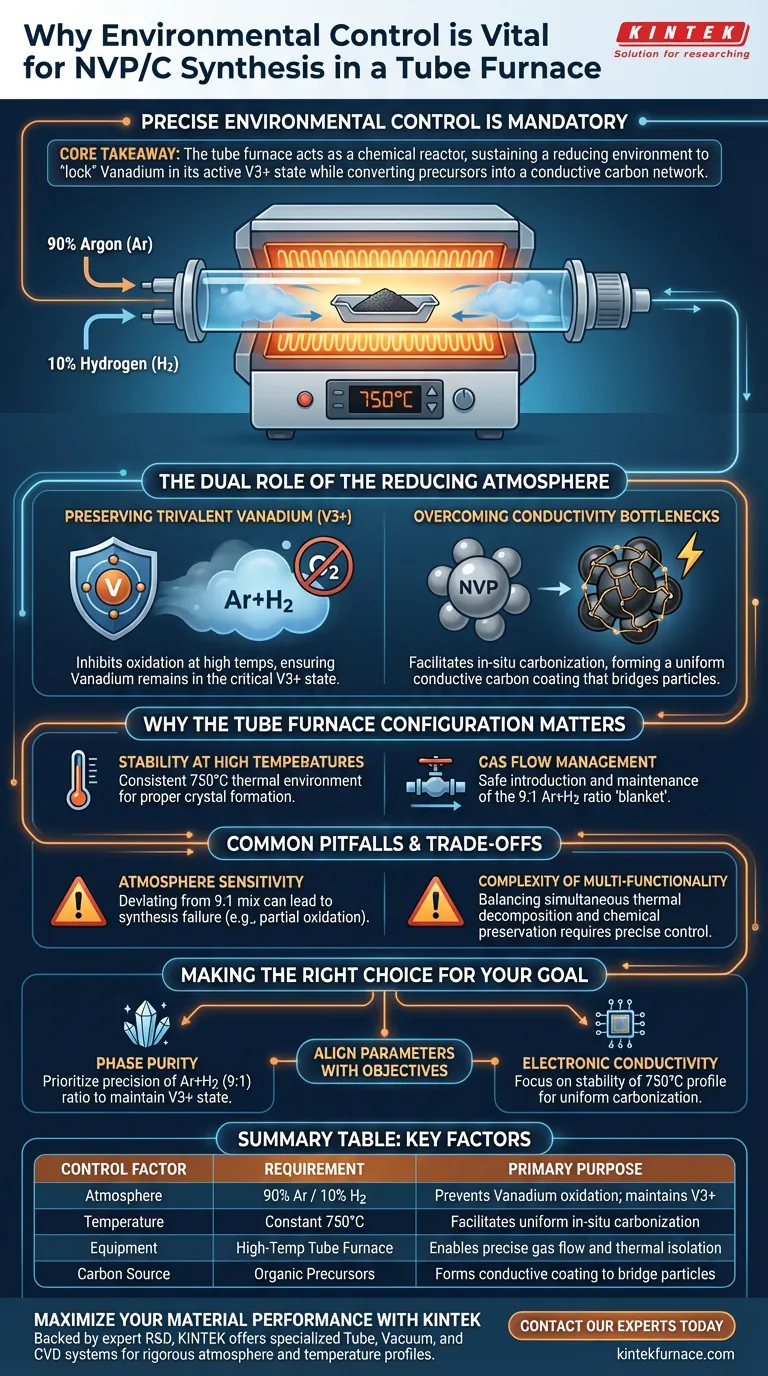
Precise environmental control is mandatory during NVP/C synthesis to simultaneously manage the chemical stability of Vanadium and the electrical properties of the final composite. Specifically, a high-temperature tube furnace maintaining a 750°C environment with a strictly controlled reducing atmosphere (90% Argon, 10% Hydrogen) is required to prevent the oxidation of Vanadium and drive the formation of a conductive carbon coating.
Core Takeaway The tube furnace acts as a chemical reactor, not just a heater. Its primary function in this context is to sustain a reducing environment that "locks" Vanadium in its active V3+ state while converting organic precursors into a conductive carbon network, directly solving the material's inherent conductivity limitations.
The Dual Role of the Reducing Atmosphere
Preserving the Trivalent Vanadium State
The primary chemical challenge in synthesizing Sodium Vanadium Phosphate (NVP) is the high reactivity of Vanadium at elevated temperatures.
Without strict environmental control, high temperatures would cause Vanadium to undergo unintended oxidation, altering its valence state.
By introducing a reducing atmosphere of Ar+H2 (9:1), the furnace inhibits this oxidation, ensuring the Vanadium remains in its critical trivalent (V3+) state.
Overcoming Conductivity Bottlenecks
NVP as a raw material suffers from low electronic conductivity, which limits its performance in electrochemical applications.
The controlled environment facilitates the in-situ carbonization of organic precursors directly onto the surface of the NVP particles.
This process forms a uniform, conductive carbon coating (the "C" in NVP/C), which bridges the particles and drastically improves the material's overall electronic conductivity.
Why the Tube Furnace Configuration Matters
Stability at High Temperatures
The synthesis requires a consistent thermal environment of 750°C to ensure proper crystal formation.
A tube furnace provides the thermal mass and isolation necessary to maintain this temperature without fluctuation, which is critical for the reaction kinetics of the carbonization process.
Gas Flow Management
Unlike standard box furnaces, a tube furnace is designed to manage the flow of specialized gases like Hydrogen.
It allows for the safe introduction and maintenance of the 9:1 Ar+H2 ratio, creating a constant "blanket" of reducing gas around the sample throughout the heat treatment.
Common Pitfalls and Trade-offs
Sensitivity to Atmosphere Composition
The specific ratio of Argon to Hydrogen is not arbitrary; deviating from the 9:1 mix can lead to synthesis failure.
Insufficient Hydrogen may result in partial oxidation of the Vanadium, while an excess could theoretically alter the reduction kinetics or pose safety risks.
The Complexity of Multi-Functionality
The furnace must achieve two competing goals simultaneously: thermal decomposition (carbonization) and chemical preservation (reduction).
If the temperature ramps up too quickly or the gas flow is inconsistent, you risk incomplete carbonization or "hot spots" where oxidation occurs despite the protective atmosphere.
Making the Right Choice for Your Goal
To ensure synthesis success, align your furnace parameters with your specific material objectives:
- If your primary focus is Phase Purity: Prioritize the precision of the Ar+H2 (9:1) ratio to rigorously exclude oxygen and maintain the V3+ state.
- If your primary focus is Electronic Conductivity: Focus on the stability of the 750°C temperature profile to ensure the organic precursors carbonize fully and uniformly over the particle surface.
Ultimately, the environmental control within the tube furnace is the deciding factor between a highly conductive, stable NVP/C composite and a non-functional, oxidized by-product.
Summary Table:
| Control Factor | Requirement | Primary Purpose |
|---|---|---|
| Atmosphere | 90% Argon / 10% Hydrogen | Prevents Vanadium oxidation; maintains V3+ state |
| Temperature | Constant 750°C | Facilitates uniform in-situ carbonization |
| Equipment | High-Temp Tube Furnace | Enables precise gas flow and thermal isolation |
| Carbon Source | Organic Precursors | Forms conductive coating to bridge particles |
Maximize Your Material Performance with KINTEK
Precise environmental control is the difference between high-performance NVP/C and synthesis failure. Backed by expert R&D and world-class manufacturing, KINTEK offers specialized Tube, Vacuum, and CVD systems designed to maintain the rigorous atmosphere and temperature profiles your research demands.
Whether you need a standard setup or a customizable high-temp furnace for unique electrochemical synthesis, our team is ready to deliver the reliability you need.
Ready to elevate your lab's capabilities? Contact our experts today to find the perfect furnace solution for your synthesis goals.
Visual Guide
References
- Madhav Sharma, R. S. Dhaka. Understanding the Electrochemical Performance and Diffusion Kinetics of HC||Na3V2(PO4)3/C Full Cell Battery for Energy Storage Applications. DOI: 10.56042/ijpap.v62i2.7371
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
People Also Ask
- What factors should be considered when purchasing a quartz tube furnace? Ensure Reliable High-Temperature Processing
- What is the purpose of pre-treating quartz tube reactors? Achieve High-Purity CVT Crystal Growth with Precision
- What role does a tubular furnace play in the synthesis of Si:B nanowires? Driving Thermal Evaporation and Growth
- What specific research applications demonstrate the capabilities of lab tubular furnaces? Unlock Precise Thermal Processing
- What are the advantages of a vacuum tube? Unlock Superior Performance in Audio & Heat Treatment
- Why is a high-performance tube furnace required for chemical activation? Achieve Precision Pore Control at 700°C
- What are tube furnaces used for? Achieve Precise Thermal Processing & Atmosphere Control
- Why is a high-vacuum sealed quartz tube used in CVT? Ensuring High-Purity Fe4GeTe2 Single Crystal Growth



















