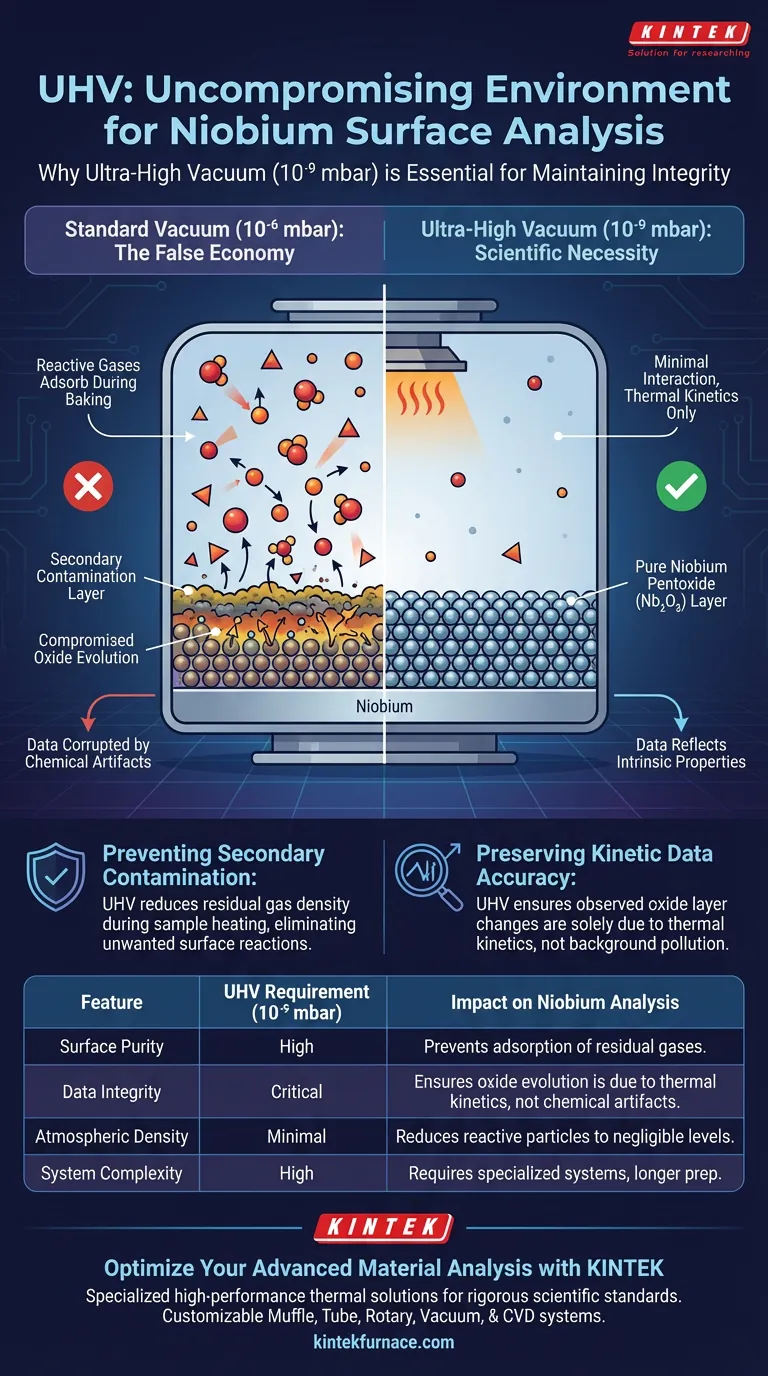
Maintaining surface integrity is the primary objective. An ultra-high vacuum (UHV) environment is required during the heat treatment and analysis of Niobium to prevent secondary contamination from residual gases. This rigorous standard is critical because Niobium surfaces are highly sensitive, and any interaction with atmospheric impurities during baking or measurement would compromise the validity of the data.
The use of base pressures at the 10^-9 mbar level is essential to ensure that the observed evolution of Niobium pentoxide layers is a result of thermal kinetics, rather than a chemical reaction with the vacuum chamber environment.
The Critical Role of Vacuum Pressure
Preventing Secondary Contamination
The primary justification for using a UHV environment is the elimination of secondary contamination.
During the baking process, the sample is heated, which can increase the reactivity of the surface. If residual gases are present in the chamber, they will adsorb onto or react with the Niobium surface.
By maintaining a pressure of 10^-9 mbar, the density of gas particles is reduced to a level where these unwanted interactions are negligible.
Preserving Kinetic Data Accuracy
The specific purpose of this analysis is often to study the evolution of very thin Niobium pentoxide (Nb2O5) layers.
Researchers need to observe how these oxide layers change strictly as a function of temperature. If the environment is not clean, external contaminants will skew the results.
UHV ensures that the kinetic data collected reflects the intrinsic properties of the Niobium and its oxide layer, rather than artifacts caused by background pollution.
Understanding the Trade-offs
Complexity vs. Data Integrity
While UHV is scientifically necessary for this application, it introduces significant operational challenges.
Achieving pressures of 10^-9 mbar requires specialized pumping systems, longer preparation times, and strict baking protocols compared to standard high vacuum systems.
However, opting for a lower quality vacuum (e.g., 10^-6 mbar) creates a false economy. The data collected in such an environment would likely be corrupted by gas adsorption, rendering the analysis of thin oxide layers scientifically invalid.
Making the Right Choice for Your Goal
When configuring your experimental setup for Niobium analysis, you must prioritize environmental purity based on your data requirements.
- If your primary focus is precise kinetic analysis: You must utilize a UHV system to ensure that temperature is the only variable affecting the oxide layer evolution.
- If your primary focus is surface purity: You need to maintain base pressures at the 10^-9 mbar level to prevent residual gases from altering the sample composition during baking.
Ultimately, the reliability of your Niobium surface analysis is directly proportional to the quality of the vacuum environment you maintain.
Summary Table:
| Feature | UHV Requirement (10^-9 mbar) | Impact on Niobium Analysis |
|---|---|---|
| Surface Purity | High | Prevents adsorption of residual gases and secondary contamination. |
| Data Integrity | Critical | Ensures oxide evolution is due to thermal kinetics, not chemical artifacts. |
| Atmospheric Density | Minimal | Reduces reactive particles to negligible levels during heating cycles. |
| System Complexity | High | Requires specialized pumping, baking protocols, and rigorous prep times. |
Optimize Your Advanced Material Analysis with KINTEK
Precise surface analysis of materials like Niobium demands an uncompromising vacuum environment. At KINTEK, we specialize in high-performance thermal solutions designed for the most rigorous scientific standards.
Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, all of which are fully customizable to meet your unique ultra-high vacuum and high-temperature needs. Whether you are studying thin oxide layers or performing complex material synthesis, our equipment provides the stability and purity required for reproducible results.
Ready to elevate your research capabilities? Contact us today to discuss how our customizable laboratory furnaces can support your next breakthrough.
Visual Guide
References
- Alena Prudnikava, Jens Knobloch. <i>In-situ</i> synchrotron x-ray photoelectron spectroscopy study of medium-temperature baking of niobium for SRF application. DOI: 10.1088/1361-6668/ad4825
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Vacuum Heat Treat Sintering and Brazing Furnace
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
People Also Ask
- Why is the water quenching process necessary for high-entropy alloys? Master Phase Purity and Microstructural Integrity
- Why is a pre-heated oxygen blowing system essential for chalcopyrite ignition? Ensure Precise Flash Smelting Simulation
- What is the function of an industrial drying oven in ZnZrOx catalyst prep? Ensure Uniform Metal Precursor Adsorption
- What is the function of the slow cooling feature in a furnace for Li2.7Sc0.1Sb? Master Single-Crystal Quality
- What role does a laboratory circulating air drying oven play in the post-treatment of composite membranes? Master Stability
- What is the function of a drying oven for oil shale semi-coke? Achieve Precise Sample Standardization
- How is an industrial high-temperature furnace utilized for beta-quench treatment of Zr-2.5%Nb alloys?
- How do high-temp furnaces influence LTO sintering? Optimize Lithium Titanate Performance via Precision Control



















