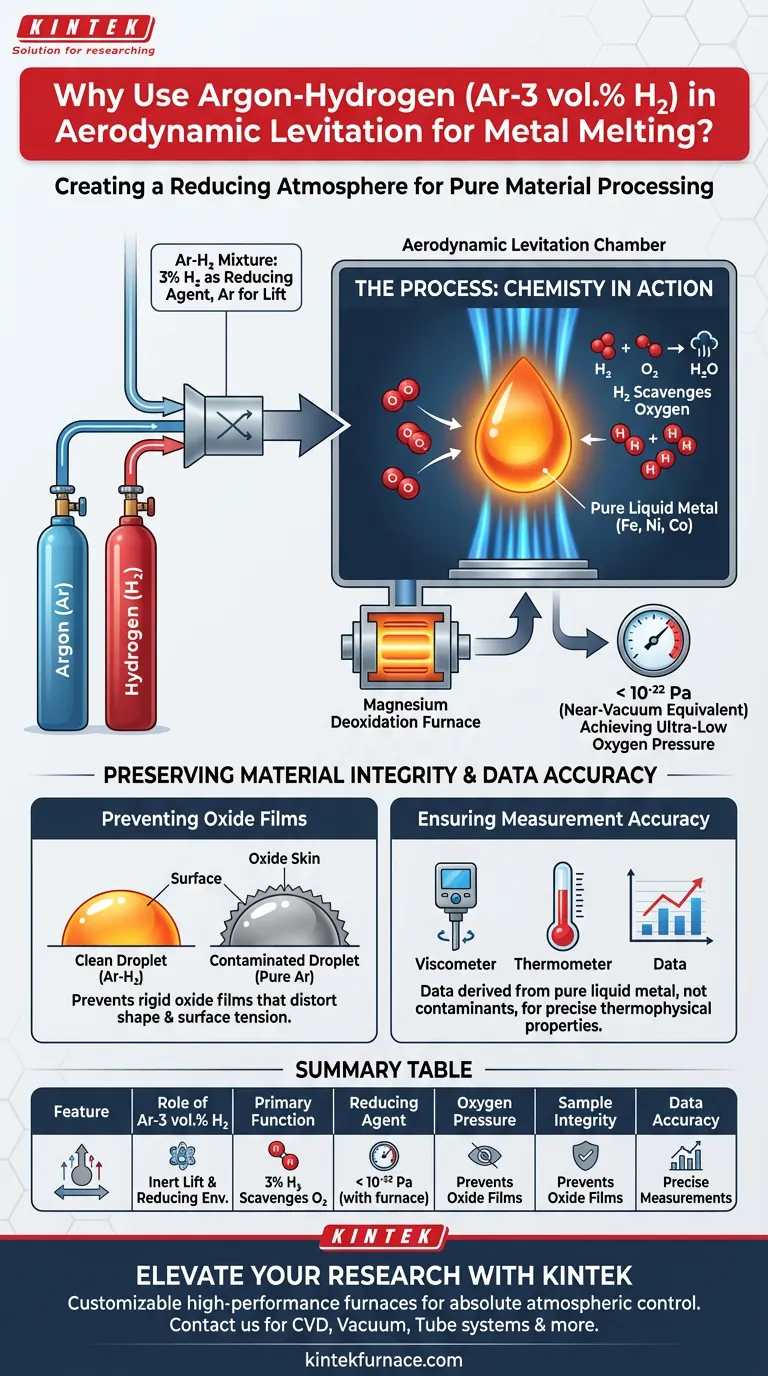
The primary function of an Argon-Hydrogen (Ar-H2) mixture in aerodynamic levitation is to chemically alter the environment around the sample to prevent oxidation. While Argon provides the inert lift force required to levitate the material, the addition of 3% Hydrogen acts as a reducing agent. This combination is essential for processing reactive metals at high temperatures without compromising their surface chemistry.
By coupling this gas mixture with a deoxidation furnace, the system lowers oxygen levels to a near-vacuum equivalent. This prevents oxide formation, ensuring that thermophysical measurements reflect the true properties of the pure metal rather than a contaminated surface.
Creating a Reducing Atmosphere
The Role of Hydrogen
Standard inert gases like pure Argon are often insufficient to prevent oxidation at the extreme temperatures required for metal melting. Even trace amounts of residual oxygen can react with the sample.
The inclusion of Hydrogen creates a reducing atmosphere. The Hydrogen actively reacts with available oxygen, effectively scavenging it from the environment before it can bond with the metal sample.
Achieving Ultra-Low Oxygen Pressure
To maximize the effectiveness of this mixture, it is often processed through a magnesium deoxidation furnace.
This additional step drives the oxygen partial pressure within the levitation chamber down to extremely low levels—specifically below 10^-22 Pa. This creates an environment that is chemically cleaner than many standard high-vacuum systems.
Preserving Material Integrity
Preventing Oxide Films
Many metals, particularly iron, nickel, and cobalt, are highly susceptible to forming oxide films immediately upon heating.
If these films form, they act as a rigid skin on the liquid droplet. This skin can distort the shape of the levitated sample or alter its surface tension, leading to instability in the levitation process.
Ensuring Measurement Accuracy
The ultimate goal of using Ar-H2 is to facilitate precise thermophysical property measurements.
When an oxide layer forms, it alters the emissivity and thermal conductivity of the sample surface. By preventing these layers, researchers ensure that the data collected—such as viscosity, density, or surface tension—is derived from the pure liquid metal, not a surface contaminant.
Operational Considerations
The Necessity of Active Deoxidation
It is important to note that simply mixing Argon and Hydrogen may not be enough for the most sensitive experiments.
The primary reference highlights that the gas mixture is processed through a magnesium deoxidation furnace to achieve the target oxygen partial pressure ($<10^{-22}$ Pa). Relying on the gas cylinder mixture alone without this active deoxidation step may not yield the extreme purity required for highly reactive transition metals.
Maximizing Experimental Success
To ensure valid data in aerodynamic levitation experiments, you must match the atmosphere to the material's reactivity.
- If your primary focus is working with Iron, Nickel, or Cobalt: You must use the Ar-H2 mixture to actively prevent the formation of surface oxide skins.
- If your primary focus is high-precision thermophysical data: Incorporate a magnesium deoxidation furnace to drive oxygen partial pressure below 10^-22 Pa, eliminating environmental interference.
The use of Argon-Hydrogen is not just about levitation; it is a critical chemical control necessary to maintain the fundamental purity of your sample.
Summary Table:
| Feature | Role of Ar-3 vol.% H2 Gas Mixture |
|---|---|
| Primary Function | Provides inert levitation force and a reducing environment |
| Reducing Agent | 3% Hydrogen scavenges residual oxygen to prevent oxidation |
| Oxygen Pressure | Achieves < 10^-22 Pa when used with a deoxidation furnace |
| Sample Integrity | Prevents oxide films on metals like Fe, Ni, and Co |
| Data Accuracy | Ensures precise measurements of surface tension and viscosity |
Elevate Your Material Research with KINTEK
Precision in aerodynamic levitation requires more than just high temperatures; it demands absolute control over your atmospheric environment. Backed by expert R&D and world-class manufacturing, KINTEK provides high-performance solutions including Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet your unique laboratory needs.
Whether you are processing reactive transition metals or seeking ultra-low oxygen partial pressures for thermophysical analysis, our advanced high-temperature furnaces ensure the purity and stability your research demands. Contact us today to optimize your thermal processes and see how our expertise can drive your experimental success.
Visual Guide
References
- Kanta Kawamoto, Hidekazu Kobatake. Development of Heat-of-fusion Measurement for Metals Using a Closed-type Aerodynamic Levitator. DOI: 10.2355/isijinternational.isijint-2024-053
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- Which gases are commonly used to create inert atmospheres in furnaces? Nitrogen vs. Argon Explained
- What are the advantages of retort? Unlock Superior Quality with Sealed Processing
- How does a precision high-temperature furnace ensure the densification of MgO? Master Low-Temp Ceramic Sintering
- How does the controlled thermal environment of a laboratory furnace support the hydrothermal synthesis of NH2-MIL-125?
- What is an atmosphere protection muffle furnace? Unlock Precise Heat Treatment in Controlled Environments
- How does the experimental box type atmosphere furnace contribute to energy conservation and environmental protection? Discover Sustainable Lab Solutions
- Why is a nitrogen-protected annealing furnace necessary for silicon steel? Preserve Magnetic Performance
- What are the key components of a retort furnace? Unlock Precise Heat Treatment Control



















