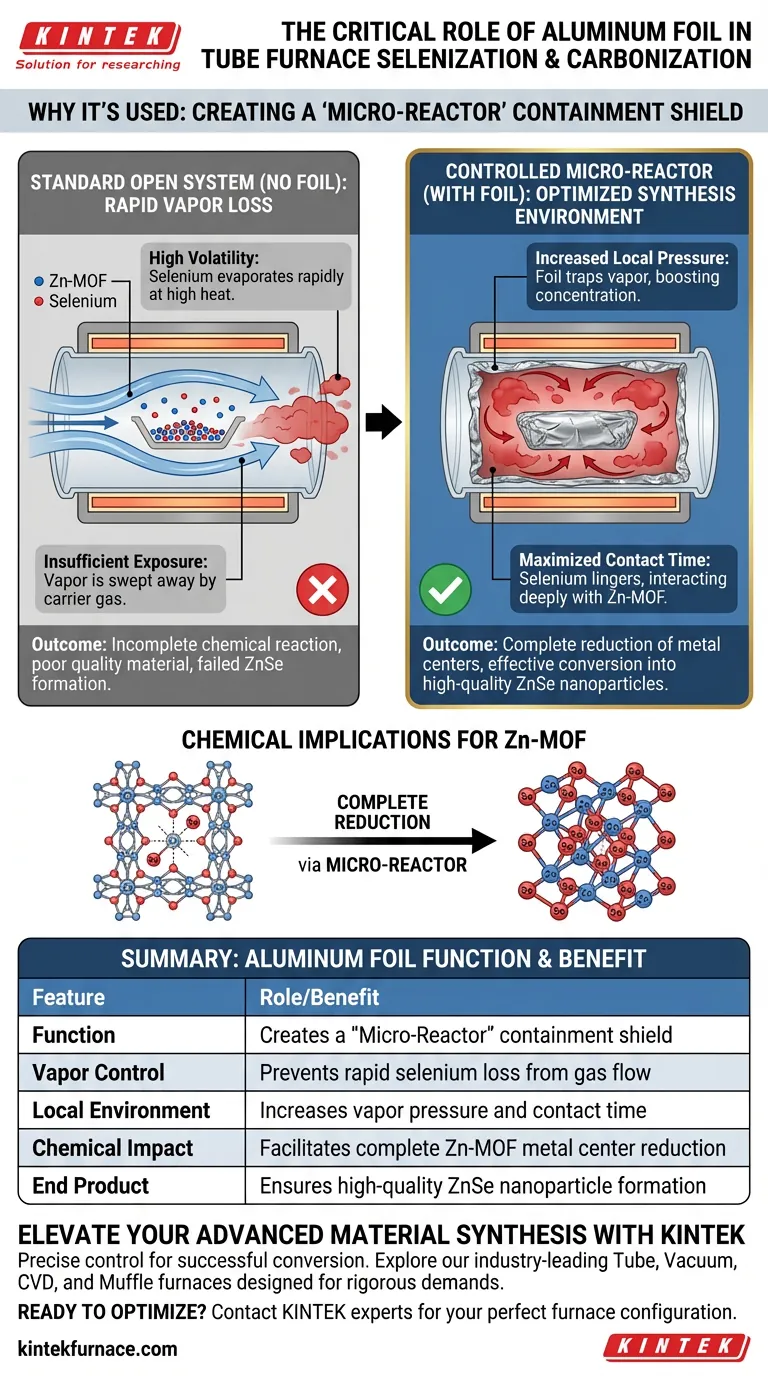
Aluminum foil functions as a critical containment shield within the tube furnace to regulate the volatility of selenium. By wrapping the precursors in foil, you transform a standard open heat treatment into a controlled, high-pressure synthesis environment that prevents reactant loss.
By acting as a "micro-reactor," the aluminum foil traps selenium vapor to increase local pressure and prolong contact time. This ensures the complete reduction of metal centers in Zn-MOF structures, facilitating their effective conversion into ZnSe nanoparticles.

The Challenge of Volatility
Managing Rapid Vapor Loss
Selenium is highly volatile at the elevated temperatures required for carbonization and selenization. In a standard tube furnace setup, the carrier gas would typically sweep these vapors away rapidly.
The Consequence of Open Systems
Without a physical barrier, the concentration of selenium vapor around the sample drops too quickly. This insufficient exposure leads to incomplete chemical reactions and poor material quality.
The "Micro-Reactor" Mechanism
Increasing Local Pressure
The aluminum foil creates a confined space around the sample, often referred to as a micro-reactor. As the selenium creates vapor, the foil traps it, significantly increasing the local vapor pressure surrounding the target material.
Maximizing Contact Time
This confinement forces the selenium vapor to linger in direct contact with the precursor material. Instead of escaping downstream, the reactant remains available to interact with the structure for the duration of the heat treatment.
Chemical Implications for Zn-MOF
Targeting Metal Centers
The primary goal of this technique is to influence the Zn-MOF (Zinc Metal-Organic Framework) structure. The trapped selenium vapor is forced to interact deeply with the metal centers of the framework.
Facilitating Complete Reduction
The high-pressure environment ensures that the metal ions are fully reduced. This intense interaction is necessary to drive the chemical conversion from a precursor state into stable ZnSe (Zinc Selenide) nanoparticles.
Understanding the Trade-offs
Containment vs. Flow
While the tube furnace provides a continuous flow of inert gas, the foil deliberately interrupts this flow at the sample level. You are prioritizing reactant density over gas exchange for the specific area of synthesis.
The Necessity of the Barrier
Omitting the foil is not merely less efficient; it often results in a failure to synthesize the target material. Without the micro-reactor effect, the conversion to ZnSe nanoparticles may be partial or nonexistent due to reactant starvation.
Making the Right Choice for Your Synthesis
To achieve high-quality semiconductor nanoparticles, applying this containment strategy is essential.
- If your primary focus is Chemical Conversion: Use the aluminum foil wrap to guarantee high local vapor pressure, ensuring the Zn-MOF precursors fully convert to ZnSe.
- If your primary focus is Vapor Management: Rely on the foil to act as a physical buffer, preventing the rapid depletion of selenium before the reaction is complete.
This simple addition transforms the thermodynamics of your furnace, ensuring your precursors react rather than evaporate.
Summary Table:
| Feature | Role of Aluminum Foil |
|---|---|
| Function | Creates a "Micro-Reactor" containment shield |
| Vapor Control | Prevents rapid selenium loss from carrier gas flow |
| Local Environment | Increases vapor pressure and reactant contact time |
| Chemical Impact | Facilitates complete reduction of Zn-MOF metal centers |
| End Product | Ensures high-quality ZnSe nanoparticle formation |
Elevate Your Advanced Material Synthesis with KINTEK
Precise atmospheric control is the difference between successful chemical conversion and failed synthesis. KINTEK provides industry-leading Tube, Vacuum, CVD, and Muffle furnaces designed to meet the rigorous demands of selenization and carbonization research.
Backed by expert R&D and world-class manufacturing, our systems offer the stability and temperature uniformity required for your most sensitive micro-reactor experiments. Whether you are developing ZnSe nanoparticles or complex MOF structures, our lab high-temp furnaces are fully customizable to your unique thermal processing needs.
Ready to optimize your lab's performance? Contact KINTEK today to consult with our experts on the perfect furnace configuration for your research goals.
Visual Guide
References
- Ying Wang, Yun Wang. <i>In‐situ</i> confining selenium within bubble – like carbon nanoshells for ultra‐stable Li−Se batteries. DOI: 10.1002/chem.202304114
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What are the primary process objectives of using an infrared belt furnace? Optimize TOPCon Solar Cell Metallization
- What is the primary role of a carbonization curing chamber? Unlock High Strength in Magnesium Slag Mortar
- What is the function of a Teflon-lined autoclave in hydrothermal acid treatment? Enhance Catalyst Synthesis Efficiency
- What is the significance of using a laboratory vacuum drying oven during the catalyst recovery phase of depolymerization?
- How does a laboratory vacuum drying oven contribute to the post-processing stage of pBN-CTF products?
- What is the primary function of a vacuum oven for Mo-based catalyst precursors? Ensure Purity & Pore Integrity
- How do continuous furnaces differ from batch furnaces? Choose the Right Furnace for Your Production Needs
- What is the role of a Pulsed Laser Deposition (PLD) system in orthopyroxene Fe-Mg experiments? Precision Film Growth



















