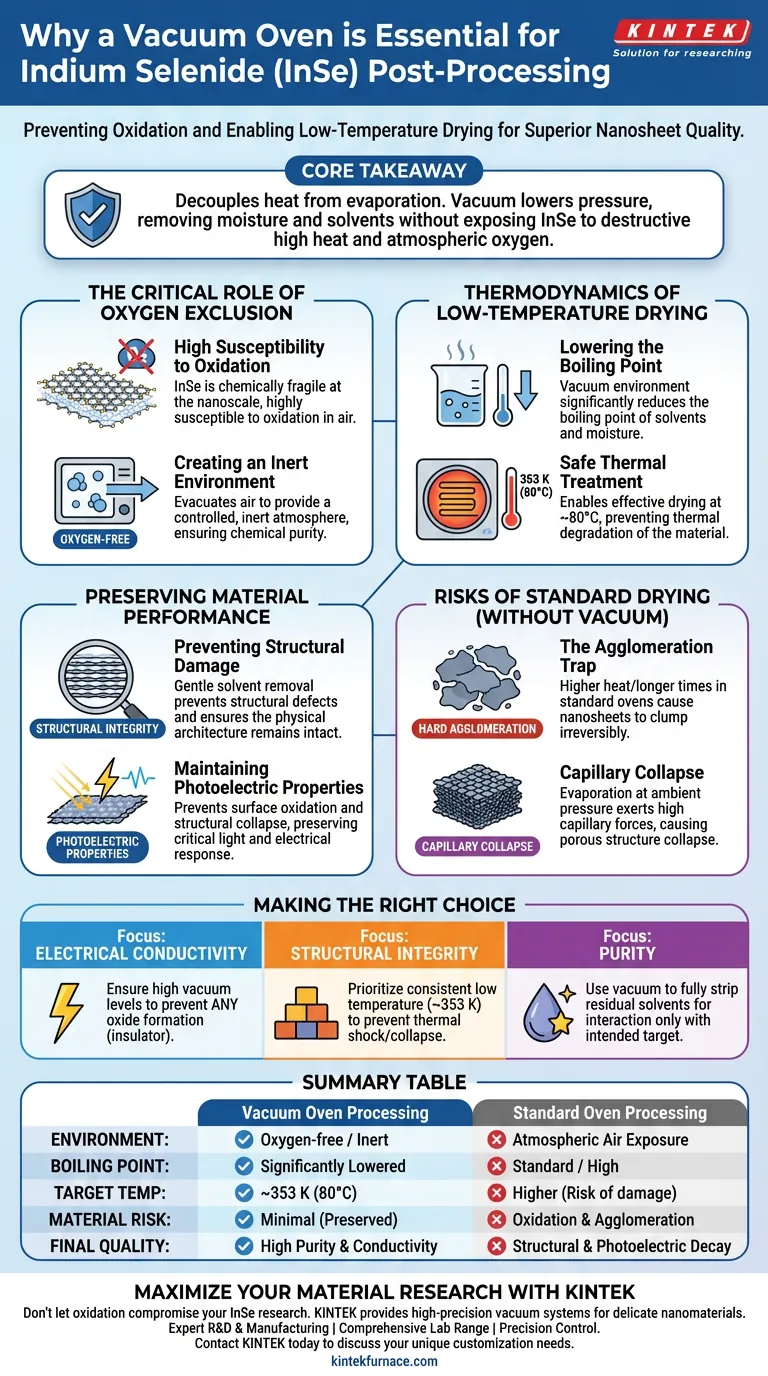
A vacuum oven is strictly required for Indium Selenide ($InSe$) post-processing to prevent oxidation while removing solvents. Because $InSe$ nanosheets are highly sensitive to oxygen, standard drying would degrade the material. The vacuum environment allows you to lower the boiling point of residual solvents, enabling effective drying at a safe, low temperature (approximately 353 K or 80°C) that preserves the material's critical photoelectric properties.
Core Takeaway Processing $InSe$ nanosheets requires decoupling heat from evaporation. By using a vacuum to lower pressure, you can remove moisture and solvents without exposing the material to the destructive combination of high heat and atmospheric oxygen.
The Critical Role of Oxygen Exclusion
High Susceptibility to Oxidation
Indium Selenide is chemically fragile at the nanoscale. The primary justification for using a vacuum oven is that $InSe$ nanosheets are highly susceptible to oxidation when exposed to air.
Creating an Inert Environment
Standard ovens circulate atmospheric air, which guarantees immediate surface degradation for $InSe$. A vacuum oven evacuates this air, providing a controlled, oxygen-free environment. This isolation is the only way to ensure the chemical composition remains pure during the drying phase.
Thermodynamics of Low-Temperature Drying
Lowering the Boiling Point
To remove residual solvents and moisture, energy is required. However, in a standard environment, the heat required to boil off solvents might damage the nanosheets. A vacuum environment significantly reduces the boiling point of these liquids.
Safe Thermal Treatment
This pressure reduction allows you to perform thermal treatment at much lower temperatures. Specifically for Indium Selenide, heating is performed at approximately 353 K (80°C). This is sufficient to evaporate residues under vacuum but cool enough to prevent thermal degradation of the material.
Preserving Material Performance
Preventing Structural Damage
High heat and surface tension can ruin nanomaterials. Vacuum drying removes solvents gently, which prevents structural damage and surface defects. This ensures the physical architecture of the nanosheet remains intact.
Maintaining Photoelectric Properties
The utility of $InSe$ often lies in its response to light and electricity. By preventing surface oxidation and structural collapse, the vacuum process directly preserves the material's photoelectric properties. Any oxide layer formed during a non-vacuum process would likely act as an insulating barrier, ruining the device's performance.
Understanding the Risks of Standard Drying
While a vacuum oven adds complexity to the workflow, attempting to bypass it leads to specific failure modes.
The Agglomeration Trap
Without vacuum, you must use higher heat or longer drying times. This often leads to hard agglomeration, where nanosheets clump together irreversibly. This reduces the effective surface area and destroys the "nano" advantage of the material.
Capillary Collapse
Evaporating solvents at ambient pressure can exert high capillary forces. This can cause the porous structure of the nanosheets to collapse. Vacuum drying minimizes these forces, keeping the structure loose and porous.
Making the Right Choice for Your Goal
If your primary focus is Electrical Conductivity: Ensure the vacuum levels are high enough to prevent any oxide formation, which acts as an insulator.
If your primary focus is Structural Integrity: Prioritize maintaining a consistent low temperature (353 K) to prevent thermal shock or capillary collapse during solvent removal.
If your primary focus is Purity: Use the vacuum phase to fully strip residual solvents, which ensures the Indium Selenide interacts only with its intended target, not leftover contaminants.
The vacuum oven is not just a drying tool; it is a preservation chamber that guarantees the functional survival of Indium Selenide nanosheets.
Summary Table:
| Feature | Vacuum Oven Processing | Standard Oven Processing |
|---|---|---|
| Environment | Oxygen-free / Inert | Atmospheric Air Exposure |
| Boiling Point | Significantly Lowered | Standard / High |
| Target Temp | ~353 K (80°C) | Higher (Risk of damage) |
| Material Risk | Minimal (Preserved) | Oxidation & Agglomeration |
| Final Quality | High Purity & Conductivity | Structural & Photoelectric Decay |
Maximize Your Material Research with KINTEK
Don't let oxidation compromise your Indium Selenide research. KINTEK provides high-precision vacuum systems designed to preserve the delicate photoelectric properties of advanced nanomaterials.
Our Value to You:
- Expert R&D & Manufacturing: Custom-engineered thermal solutions for sensitive materials.
- Comprehensive Lab Range: From Muffle and Tube furnaces to specialized Vacuum and CVD systems.
- Precision Control: Maintain the exact 353 K environment required for InSe post-processing.
Ready to elevate your lab's performance? Contact KINTEK today to discuss your unique customization needs.
Visual Guide
References
- Yi Xu, Wei Feng. Photoelectrochemical-Type Photodetectors Based on Ball Milling InSe for Underwater Optoelectronic Devices. DOI: 10.3390/nano15010003
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Sintering and Brazing Furnace
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
- Vacuum Induction Melting Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- What is the function of a vacuum sintering furnace in CoNiCrAlY coatings? Repairing Cold-Sprayed Microstructures
- What is the maximum operating temperature for molybdenum in vacuum furnaces? Key to High-Temp Processing
- How do advancements in graphite coatings improve vacuum furnace components? Enhance Purity and Durability
- How are vacuum furnaces environmentally friendly? Achieve Clean, Efficient Heat Treatment
- What role does hydrogen play in the operation of a vacuum sintering furnace? Unlock Superior Sintering Quality and Efficiency
- How is a vacuum furnace energy-efficient? Uncover Key Mechanisms for Lower Costs
- What are the advantages of vacuum carburizing over conventional atmosphere-carburizing methods? Boost Quality and Efficiency in Case Hardening
- Why is Ni-25Cr-6P-1.5Si-0.5B-1.5Mo amorphous brazing foil selected? Optimize Brazing Efficiency & Quality



















