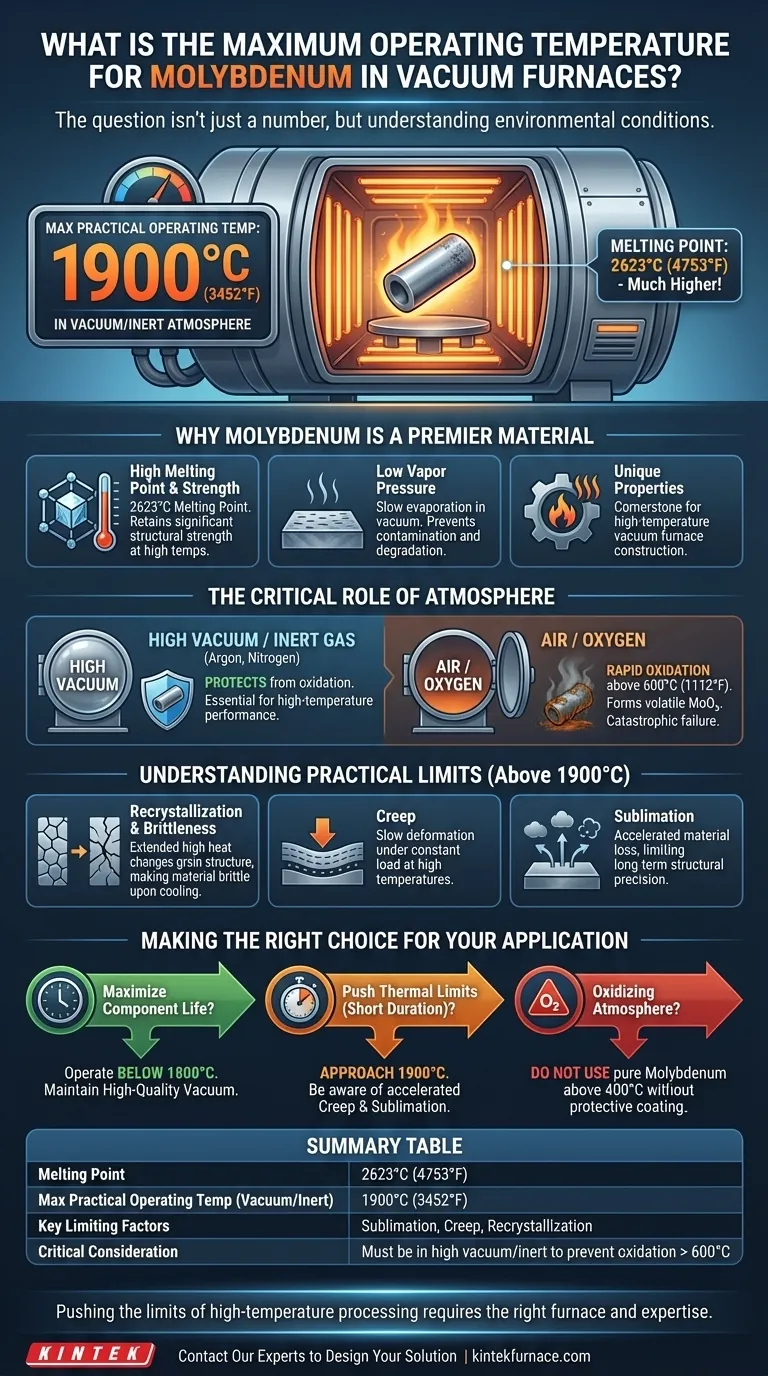
In a vacuum or inert atmosphere, the maximum practical operating temperature for pure molybdenum is typically considered to be 1900°C (3452°F). Above this point, factors like sublimation and creep become significant concerns, even though its melting point is much higher at 2623°C.
The question isn't just about a single temperature number, but about understanding the environmental conditions that allow molybdenum to reach its potential. The presence of even trace amounts of oxygen drastically reduces its maximum operating temperature.

Why Molybdenum is a Premier High-Temperature Material
Molybdenum is a cornerstone material for high-temperature vacuum furnace construction due to its unique combination of properties.
High Melting Point and Strength
Molybdenum's exceptionally high melting point of 2623°C (4753°F) gives it a massive thermal runway. More importantly, it retains significant structural strength at temperatures where many other metals would fail.
Low Vapor Pressure
Even at very high temperatures, molybdenum evaporates (sublimes) very slowly. This is critical in a vacuum environment, as it prevents the material from degrading and contaminating the furnace or the workload.
The Critical Role of the Atmosphere
The "maximum temperature" of molybdenum is entirely dependent on its surrounding environment. Its performance in a vacuum is drastically different from its performance in air.
The Threat of Oxidation
Molybdenum's primary weakness is its poor resistance to oxidation at high temperatures. In the presence of oxygen, it begins to rapidly oxidize and form molybdenum trioxide (MoO₃) at temperatures as low as 600°C (1112°F).
This oxide is volatile and sublimes away, leading to a rapid loss of material, a phenomenon sometimes called "catastrophic oxidation."
The Importance of High Vacuum
A high-quality vacuum or a pure, inert gas atmosphere (like argon or nitrogen) is essential to protect molybdenum from oxygen. The lower the pressure (the better the vacuum), the fewer oxygen molecules are present to react with the hot metal surfaces.
Even a poor vacuum can contain enough residual oxygen to cause significant damage over time, drastically lowering the effective service temperature.
Understanding the Practical Limits
While the theoretical melting point is over 2600°C, practical engineering considerations set a lower, safer limit.
Recrystallization and Brittleness
When held at high temperatures for extended periods, molybdenum's grain structure changes through a process called recrystallization. This process can make the material more brittle and prone to fracture once it cools back to room temperature.
The design of furnace components must account for this change in mechanical properties to ensure long-term reliability.
Creep and Sublimation
Creep is the tendency of a material to slowly deform under a constant load at high temperatures. Sublimation is the direct transition from a solid to a gas.
While molybdenum has excellent resistance to both, these effects become more pronounced above 1900°C, limiting its use for structural components that must maintain precise dimensions over thousands of hours.
Making the Right Choice for Your Application
Your operational parameters will determine how you use molybdenum effectively.
- If your primary focus is maximizing component life: Operate below 1800°C and ensure you maintain a high-quality vacuum to prevent any form of oxidation.
- If your primary focus is pushing thermal limits for short durations: You can approach the 1900°C limit, but be aware that this accelerates creep and sublimation, potentially reducing the component's service life.
- If your process involves any oxidizing atmosphere: Do not use pure molybdenum above 400°C without a protective coating, as rapid degradation will occur.
Understanding these environmental dependencies is the key to leveraging molybdenum's incredible high-temperature capabilities safely and effectively.
Summary Table:
| Property | Value / Key Insight |
|---|---|
| Melting Point | 2623°C (4753°F) |
| Max Practical Operating Temp (Vacuum/Inert) | 1900°C (3452°F) |
| Key Limiting Factors | Sublimation, Creep, Recrystallization |
| Critical Consideration | Must be in a high vacuum or inert atmosphere to prevent catastrophic oxidation above 600°C |
Pushing the limits of high-temperature processing requires the right furnace and expertise. KINTEK's advanced high-temperature vacuum furnaces, including our Muffle, Tube, and Atmosphere Furnaces, are engineered with materials like molybdenum to reliably meet your most demanding thermal requirements. Our strong in-house R&D and manufacturing capabilities allow for deep customization, ensuring your furnace solution is precisely tailored for your unique application, materials, and temperature goals. Contact us today to discuss how we can help you achieve superior results safely and efficiently.
Contact Our Experts to Design Your Solution
Visual Guide
Related Products
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- Molybdenum Vacuum Heat Treat Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
People Also Ask
- What are the advantages of a vacuum hot pressing sintering furnace for rare earth copper composites? Density & Purity
- What is the mechanism of a vacuum sintering furnace for AlCoCrFeNi2.1 + Y2O3? Optimize Your High-Entropy Alloy Processing
- How do vacuum sintering and annealing furnaces contribute to the densification of NdFeB magnets?
- What is the function of a vacuum sintering furnace in CoNiCrAlY coatings? Repairing Cold-Sprayed Microstructures
- Why is a vacuum environment essential for sintering Titanium? Ensure High Purity and Eliminate Brittleness



















