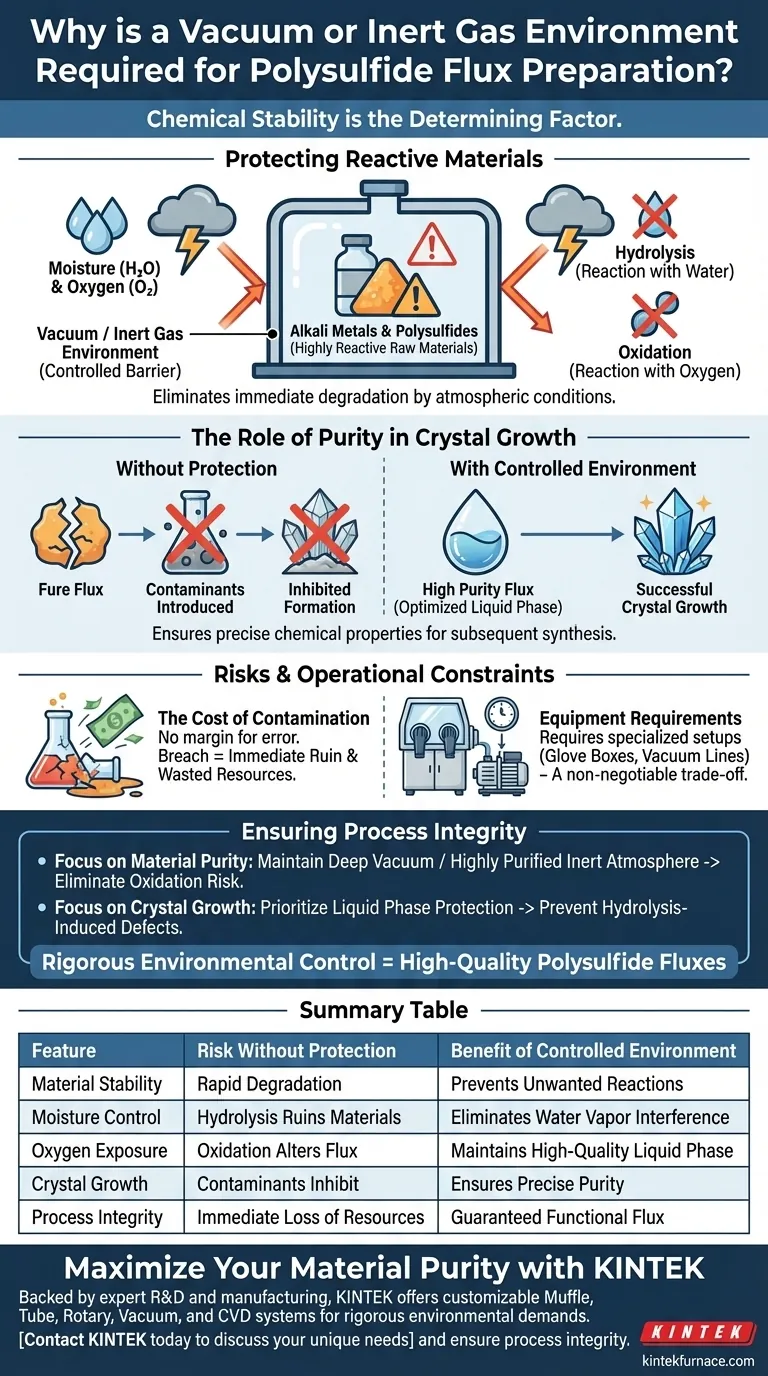
Chemical stability is the determining factor. The preparation of polysulfide fluxes requires a vacuum or inert gas environment to protect the raw materials—specifically alkali metals and their polysulfides—from immediate degradation. These substances are highly reactive to atmospheric conditions; isolating them prevents unwanted chemical reactions, ensuring the final product remains pure.
Alkali metals and polysulfides degrade rapidly when exposed to air. A controlled environment eliminates moisture and oxygen, preventing hydrolysis and oxidation to ensure a high-quality liquid phase essential for successful crystal growth.

Protecting Reactive Materials
The Sensitivity of Alkali Metals
Alkali metals and their associated polysulfides possess extreme chemical sensitivity. They are not stable in standard atmospheric conditions and react aggressively upon contact with air.
Blocking Moisture and Oxygen
The two primary environmental threats to these materials are moisture and oxygen. Utilizing a vacuum or inert gas environment acts as a total barrier, physically separating the raw materials from these reactive elements.
Preventing Chemical Degradation
Without strict isolation, the raw materials undergo hydrolysis (reaction with water) or oxidation (reaction with oxygen). These reactions alter the fundamental chemistry of the flux, rendering it unsuitable for its intended application.
The Role of Purity in Crystal Growth
Creating a Pure Flux
The ultimate objective of the preparation process is to generate a flux of high purity. Any exposure to air introduces contaminants that compromise the integrity of the flux before the growth process even begins.
Optimizing the Liquid Phase
Successful crystal growth relies heavily on the quality of the liquid phase. By preventing hydrolysis and oxidation, the controlled environment ensures this phase maintains the precise chemical properties required for subsequent synthesis.
Risks and Operational Constraints
The Cost of Contamination
There is virtually no margin for error regarding environmental exposure. If the vacuum or inert seal is breached, the resulting oxidation or hydrolysis typically ruins the raw materials immediately, wasting resources and time.
Equipment Requirements
Achieving this environment requires specialized equipment, such as glove boxes or vacuum lines. While this adds complexity to the experimental setup, it is a non-negotiable trade-off required to obtain a functional flux.
Ensuring Process Integrity
To achieve high-quality results, you must view environmental control as a critical processing step, not just a safety precaution.
- If your primary focus is Material Purity: Ensure your equipment can maintain a deep vacuum or a highly purified inert atmosphere to completely eliminate the risk of oxidation.
- If your primary focus is Crystal Growth: Prioritize the protection of the liquid phase during preparation to prevent hydrolysis-induced defects from inhibiting crystal formation.
Rigorous environmental control is the only way to successfully transform reactive alkali metals into high-quality polysulfide fluxes.
Summary Table:
| Feature | Risk Without Protection | Benefit of Controlled Environment |
|---|---|---|
| Material Stability | Rapid degradation of alkali metals | Prevents unwanted chemical reactions |
| Moisture Control | Hydrolysis ruins raw materials | Eliminates water vapor interference |
| Oxygen Exposure | Oxidation alters flux chemistry | Maintains high-quality liquid phase |
| Crystal Growth | Contaminants inhibit formation | Ensures precise purity for synthesis |
| Process Integrity | Immediate loss of resources | Guaranteed functional flux production |
Maximize Your Material Purity with KINTEK
Don't let atmospheric contamination ruin your research. Backed by expert R&D and manufacturing, KINTEK offers specialized Muffle, Tube, Rotary, Vacuum, and CVD systems—all customizable to meet the rigorous environmental demands of polysulfide flux preparation.
Whether you need deep vacuum capabilities or high-purity inert gas controls, our lab high-temp furnaces provide the stability required for successful crystal growth and material synthesis. Contact KINTEK today to discuss your unique needs and ensure your process integrity with our precision-engineered solutions.
Visual Guide
References
- С.А. Новиков, Vladislav V. Klepov. Structural evolution and bonding features of electron deficient copper chalcogenides. DOI: 10.1039/d5ce00479a
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
People Also Ask
- What is the main purpose of an atmosphere control during heat treating? Ensure Consistent Metallurgical Properties
- What are the technical advantages of a Zero-reforming Vertical Furnace? Revolutionize Green DRI Production Today
- What role does airflow play in maintaining furnace atmospheres? Optimize Heat Treatment Quality
- Why is a sealed environment important in a controlled atmosphere furnace? Ensure Precision and Safety in High-Temp Processes
- What is the purpose of a chemically reactive atmosphere in material processing? Achieve Precise Surface Modification for Enhanced Performance
- What are the key benefits of using argon in furnaces? Ensure Maximum Purity and Performance
- Which factors influence the radial equivalent thermal conductivity of steel coils? Key Impacts on Annealing Efficiency
- What are the key aspects of a reducing atmosphere in furnace operations? Master Heat Treatment for Superior Results



















