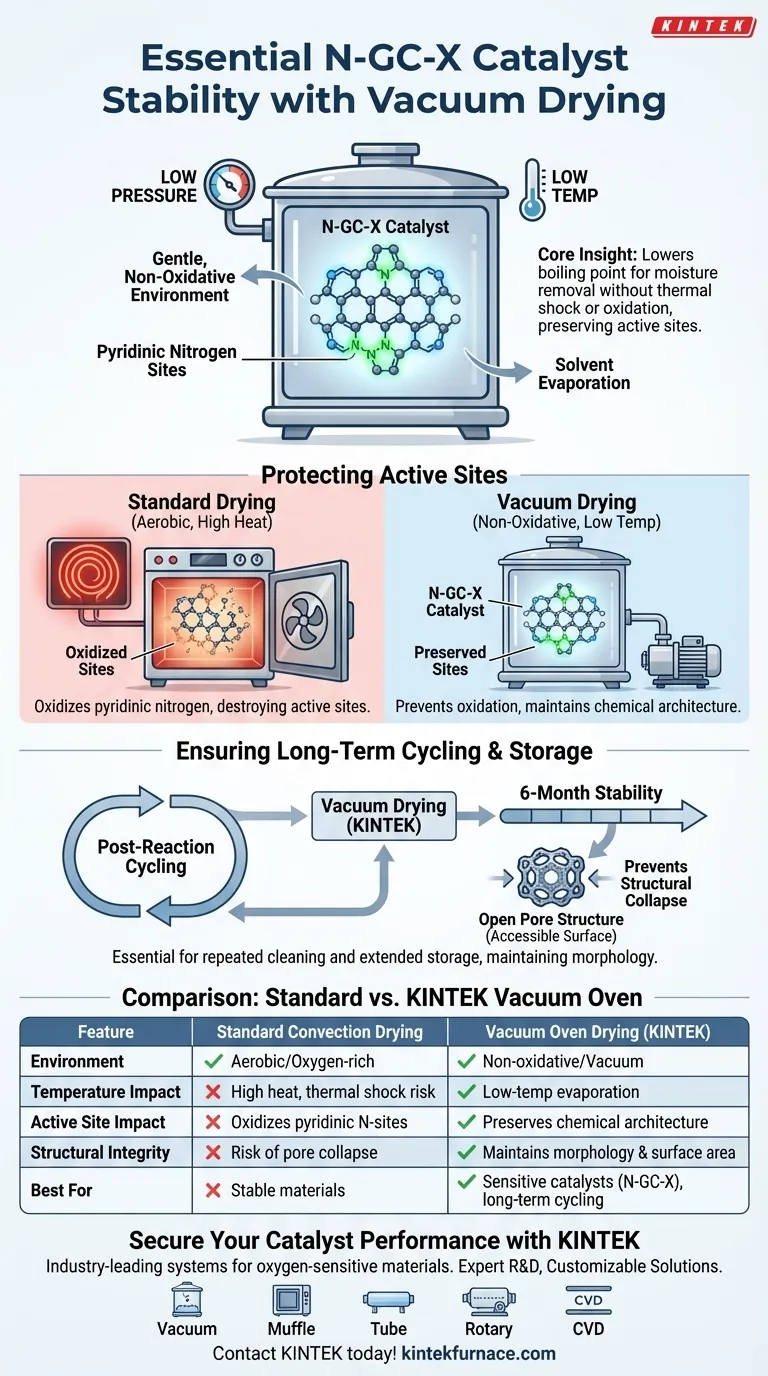
The stability of N-GC-X catalysts relies fundamentally on the gentle, non-oxidative environment of a vacuum drying oven. This equipment is strictly necessary to remove residual solvents and moisture at low temperatures, specifically protecting oxygen-sensitive pyridinic nitrogen sites from structural degradation that occurs in high-temperature, aerobic environments.
Core Insight By lowering the boiling point of solvents, vacuum drying facilitates moisture removal without subjecting the catalyst to thermal shock or oxidation. This preserves the specific chemical architecture of the active sites, ensuring consistent performance over long-term storage and repeated reaction cycles.

Protecting the Active Sites
The Vulnerability of Pyridinic Nitrogen
The N-GC-X catalyst derives its activity from specific structural features, most notably pyridinic nitrogen sites.
These sites are highly sensitive to oxygen, particularly when combined with heat.
preventing Oxidative Degradation
Standard drying methods often rely on high heat in an open atmosphere.
For N-GC-X catalysts, this exposure would lead to the oxidation of the pyridinic nitrogen, effectively destroying the active sites and reducing catalytic performance.
The Role of Reduced Pressure
A vacuum drying oven operates by significantly reducing the pressure surrounding the sample.
This allows water and solvents to evaporate at much lower temperatures than they would at standard atmospheric pressure.
Ensuring Long-Term Cycling Stability
Post-Reaction Preservation
The catalyst must be dried after each reaction cycle to remove contaminants.
Using a vacuum oven ensures that this repetitive cleaning process does not cumulatively damage the material structure.
Six-Month Stability Benchmarks
Primary data indicates that this drying protocol is essential for maintaining stability over extended periods, such as a six-month test cycle.
Without this intervention, the gradual degradation of active sites would render long-term storage data unreliable.
Preventing Structural Collapse
Beyond chemical protection, vacuum drying helps maintain the physical morphology of the catalyst.
Gentle evaporation prevents the collapse of pore structures, which is critical for maintaining the accessible surface area required for future reactions.
Operational Considerations and Trade-offs
Equipment Complexity vs. Sample Integrity
While vacuum drying requires more complex equipment (pumps and seals) than standard convection ovens, it is the only viable option for oxygen-sensitive materials.
The trade-off is a slight increase in operational complexity in exchange for non-negotiable chemical preservation.
Batch Processing Limitations
Vacuum drying is inherently a batch process, which can limit throughput compared to continuous drying methods.
However, for high-value catalysts like N-GC-X, the priority is material quality over processing speed.
Making the Right Choice for Your Goal
To ensure the validity of your catalyst research, apply the following protocols:
- If your primary focus is long-term stability: Strictly adhere to vacuum drying after every single reaction cycle to prevent cumulative oxidative damage to pyridinic nitrogen sites.
- If your primary focus is structural morphology: Use the vacuum setting to lower the drying temperature, preventing pore collapse and ensuring the powder remains loose and accessible.
Ultimately, the vacuum drying oven is not just a drying tool; it is a preservation chamber essential for the survival of the N-GC-X catalyst's active sites.
Summary Table:
| Feature | Standard Convection Drying | Vacuum Oven Drying (KINTEK) |
|---|---|---|
| Drying Environment | Aerobic (Oxygen-rich) | Non-oxidative (Vacuum) |
| Temperature Impact | High heat; risk of thermal shock | Low-temperature solvent evaporation |
| Active Site Impact | Oxidizes pyridinic nitrogen sites | Preserves chemical architecture |
| Structural Integrity | Risk of pore collapse | Maintains morphology and surface area |
| Best For | Stable, non-sensitive materials | Sensitive catalysts (N-GC-X), long-term cycling |
Secure Your Catalyst Performance with KINTEK
Don't compromise your N-GC-X research with subpar drying methods. KINTEK provides industry-leading vacuum drying systems designed to protect oxygen-sensitive active sites and maintain the structural integrity of your high-value materials. Backed by expert R&D and manufacturing, KINTEK offers Vacuum, Muffle, Tube, Rotary, and CVD systems, all customizable for your unique lab requirements.
Ensure your catalysts survive long-term cycling and storage—contact KINTEK today to find the perfect high-temperature solution for your lab!
Visual Guide
References
- Ganchang Lei, Lilong Jiang. Atom-economical insertion of hydrogen and sulfur into carbon–nitrogen triple bonds using H<sub>2</sub>S <i>via</i> synergistic C–N sites. DOI: 10.1039/d5ey00110b
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 1200℃ Muffle Oven Furnace for Laboratory
- Vacuum Heat Treat Sintering and Brazing Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- Why is Spark Plasma Sintering (SPS) optimal for Ti2AlN ceramics? Achieving 99.2% Purity and Maximum Density
- What materials are used in a vacuum furnace? Key Components for Extreme Heat & Purity
- Why is a vacuum drying oven required for Se/PPS composite treatment at 110°C? Ensure Chemical Purity & Bond Strength
- What advantages does the non-linear processing in a vacuum furnace offer? Achieve Precise Material Control
- What are the main advantages of continuous furnaces? Boost Efficiency and Cut Costs in Mass Production
- What are the advantages of using a vacuum brazing furnace over other metal joining processes? Achieve Clean, Strong, and Distortion-Free Metal Joints
- How does a vacuum furnace facilitate precise control of tellurium vacancy concentrations in PtTe2 thin films?
- What role does an industrial high-temperature vacuum furnace play in the post-treatment of semiconductor nanocrystals?



















