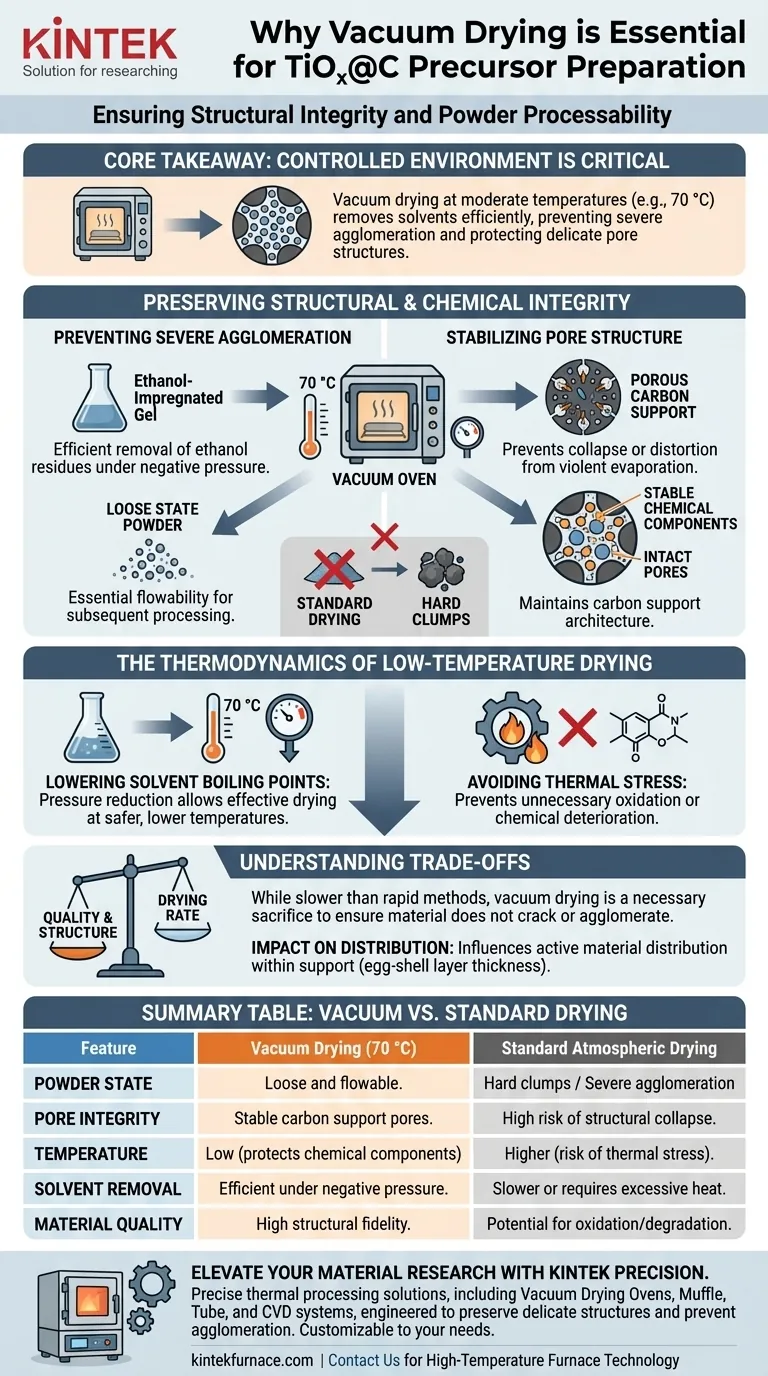
The preparation of TiOx@C precursors requires a vacuum drying oven to efficiently remove solvents like ethanol at moderate temperatures (specifically around 70 °C) without damaging the composite structure. This controlled environment is critical for preventing severe agglomeration of the material and maintaining the stability of chemical components nestled within the carbon support pores, ensuring the final powder remains loose and workable.
Core Takeaway Vacuum drying allows for solvent evaporation at reduced pressures, which significantly lowers the required temperature for drying. This protects the TiOx@C precursor from structural collapse and agglomeration, preserving the integrity of the carbon pores and ensuring the material remains in a loose, high-quality state.

Preserving Structural and Chemical Integrity
Preventing Severe Agglomeration
For TiOx@C precursors, the physical state of the final powder is paramount. Standard drying methods often lead to particles sticking together, forming hard clumps.
A vacuum drying oven prevents this severe agglomeration. By removing solvents gently under negative pressure, the process yields a precursor powder that remains in a loose state. This flowability is essential for any subsequent processing steps.
Stabilizing Pore Structure
The "C" in TiOx@C refers to a carbon support, which relies on a specific porous architecture to function effectively.
The vacuum environment maintains the stability of chemical components within these carbon support pores. It prevents the collapse or distortion of the internal structure that can occur when solvents evaporate violently or at high temperatures.
Handling Ethanol-Impregnated Gels
The precursor often begins as an ethanol-impregnated gel mixture.
Vacuum drying is uniquely quality-suited for this phase because it enables the efficient removal of ethanol residues. It ensures the gel transitions to a solid powder without trapping solvents that could interfere with later pyrolysis or sintering stages.
The Thermodynamics of Low-Temperature Drying
Lowering Solvent Boiling Points
The fundamental advantage of this method is the relationship between pressure and temperature. By reducing the pressure inside the oven, the boiling point of solvents like ethanol drops significantly.
This allows for effective drying at 70 °C, a temperature that is safe for the precursor but would be inefficient for solvent removal at standard atmospheric pressure.
Avoiding Thermal Stress
Heat is often the enemy of delicate organic-inorganic hybrids.
By keeping the process temperature low (e.g., 70 °C), you avoid the risk of unnecessary oxidation or chemical deterioration. This ensures the TiOx species does not undergo unwanted phase changes or degradation before the controlled heating of the final synthesis steps.
Understanding the Trade-offs
Drying Rate vs. Quality
While vacuum drying provides superior structural preservation, it is generally slower than rapid convective drying techniques.
The reduced pressure mitigates deep penetration of liquids, but the rate of moisture removal is more gradual. This is a necessary sacrifice to ensure the material does not crack or agglomerate, but it requires more patience than blast drying.
Impact on Distribution
The drying method influences how active materials are distributed within the support.
Vacuum drying typically results in an active material distribution (often referred to as egg-shell layer thickness) that falls somewhere between normal oven drying and rapid drying. You must ensure this specific distribution profile aligns with your electrochemical performance goals.
Making the Right Choice for Your Goal
To optimize the preparation of your TiOx@C precursors, consider your specific priorities:
- If your primary focus is Structural Fidelity: Prioritize vacuum drying to prevent pore collapse and ensure the chemical components remain stable inside the carbon support.
- If your primary focus is Powder Processability: Use vacuum drying to guarantee a loose, non-agglomerated powder that is easy to handle in subsequent steps.
Vacuum drying is not just a drying step; it is a structural preservation strategy that defines the quality of your final composite material.
Summary Table:
| Feature | Vacuum Drying (70 °C) | Standard Atmospheric Drying |
|---|---|---|
| Powder State | Loose and flowable | Hard clumps / Severe agglomeration |
| Pore Integrity | Stable carbon support pores | High risk of structural collapse |
| Temperature | Low (protects chemical components) | Higher (risk of thermal stress) |
| Solvent Removal | Efficient under negative pressure | Slower or requires excessive heat |
| Material Quality | High structural fidelity | Potential for oxidation/degradation |
Elevate Your Material Research with KINTEK Precision
Precise thermal processing is the foundation of high-performance TiOx@C composites. KINTEK provides industry-leading lab solutions, including Vacuum Drying Ovens, Muffle, Tube, and CVD systems, specifically engineered to preserve delicate pore structures and prevent agglomeration.
Backed by expert R&D and manufacturing, our equipment is fully customizable to meet the unique demands of your advanced materials synthesis. Don't compromise on structural integrity—contact us today to discover how our high-temperature furnace technology can optimize your lab's workflow and results.
Visual Guide
References
- Zihan Wei, Guisheng Li. Highly Dispersed Pt on TiOx Embedded in Porous Carbon as Electrocatalyst for Hydrogen Evolution Reaction. DOI: 10.3390/catal15050487
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Sintering and Brazing Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
People Also Ask
- What are the technical advantages of using a high-vacuum high-temperature sintering furnace for stainless steel?
- What is the primary purpose of using a vacuum drying oven for rGO/tMO composites? Ensure Purity and Structural Integrity
- How do heat treatment and vacuum furnaces contribute to industrial innovation? Unlock Superior Material Performance
- What factors should be considered when selecting a vacuum arc furnace? Optimize Your Material Production with Expert Insights
- How precise is the temperature measurement and monitoring in a vacuum furnace? Achieve ±1.5°C Accuracy and ±5°C Uniformity
- What is the primary function of the vacuum system in the vacuum distillation process for metal purification? Achieve High-Purity Metal Separation
- How are vacuum coating furnaces applied in the semiconductor and electronic components industry? Essential for High-Purity Electronics
- How does the high-power rapid scanning preheating cycle affect material quality? Stabilize PBF-EB & Prevent Cracking



















