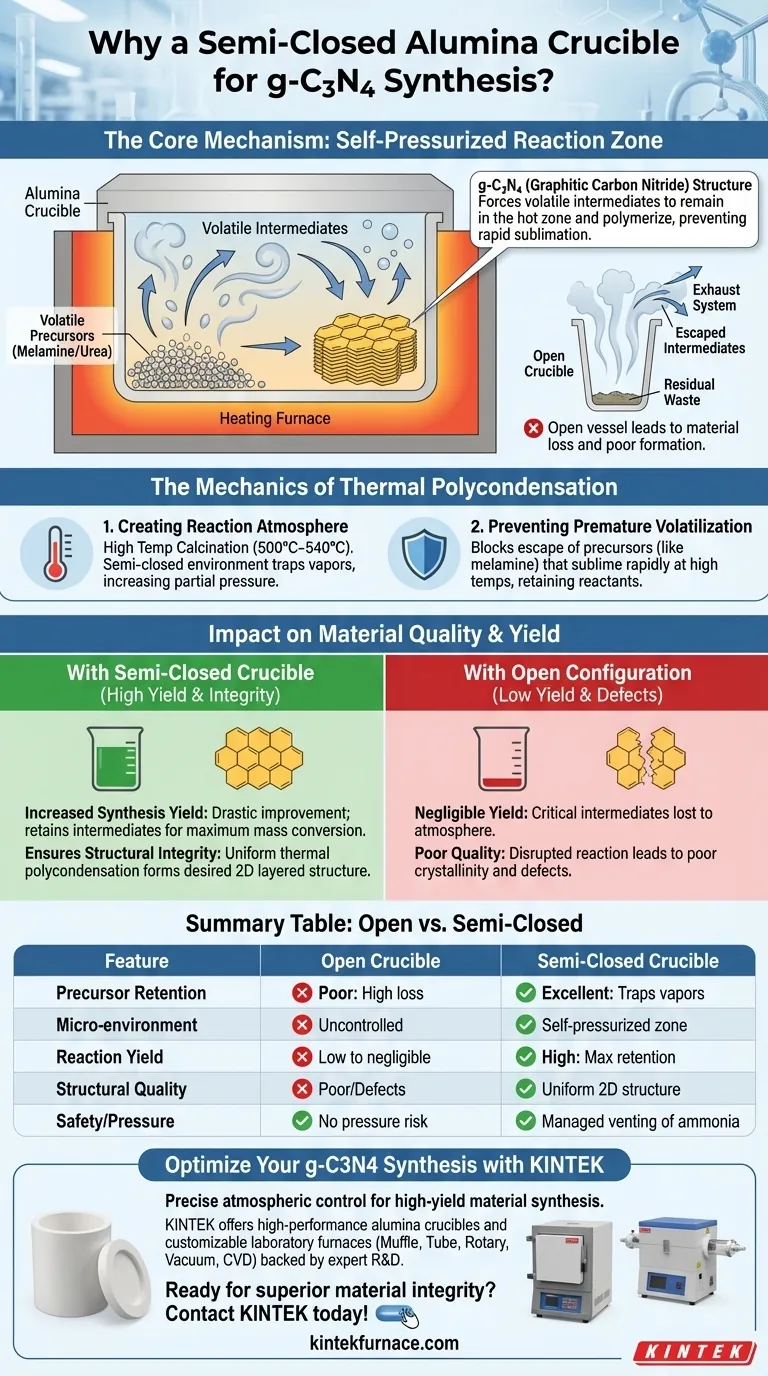
A semi-closed alumina crucible is mandatory during g-C3N4 synthesis because it creates a confined micro-environment that prevents the rapid sublimation of volatile precursors. Without a lid, materials like melamine or urea would evaporate and escape the vessel before they could undergo the necessary thermal polycondensation to form the final graphitic carbon nitride structure.
The Core Mechanism: The lid creates a "self-pressurized" zone that forces volatile intermediates to remain in the hot zone and polymerize rather than simply evaporating away. This mechanical constraint is the deciding factor between obtaining a high-yield, high-quality powder or an empty crucible.

The Mechanics of Thermal Polycondensation
Creating the Necessary Reaction Atmosphere
The synthesis of g-C3N4 involves calcining precursors such as melamine or urea at high temperatures (typically 500°C–540°C).
During this process, the material does not simply melt and react; it decomposes into various gas-phase and solid-phase intermediates.
A semi-closed environment (a crucible with a lid) traps these vapors. This increases the partial pressure of the intermediates, forcing them to interact and polymerize into the desired graphitic structure.
Preventing Premature Volatilization
Precursors like melamine are highly volatile at elevated temperatures.
In an open vessel, these materials would sublime (turn directly from solid to gas) and vent into the exhaust system long before forming the stable g-C3N4 lattice.
By using a lid, you physically block this escape route, retaining the reactants within the heating zone.
Impact on Material Quality and Yield
Increasing Synthesis Yield
The most immediate benefit of the semi-closed crucible is a drastic improvement in yield.
The primary reference notes that this specific environment prevents the excessive volatilization of intermediate products.
By retaining these intermediates, the system ensures that the majority of the starting mass is converted into the final product rather than being lost as waste vapor.
Ensuring Structural Integrity
Beyond simple mass retention, the semi-closed atmosphere dictates the quality of the crystal lattice.
The confined environment facilitates a uniform thermal polycondensation reaction.
According to supplementary data, this micro-environment helps ensure the final light-yellow powder possesses the desired two-dimensional layered structure characteristic of high-quality graphitic carbon nitride.
Understanding the Trade-offs
The "Semi-Closed" Distinction
It is critical to note the requirement is for a semi-closed system, not a hermetically sealed one.
The polymerization process releases byproducts, such as ammonia gas, which must be allowed to vent gradually.
A completely sealed vessel (like an autoclave) could lead to dangerous pressure buildup or inhibit the reaction equilibrium by trapping waste gases.
Risks of an Open Configuration
Conversely, omitting the lid entirely is a common failure point in g-C3N4 synthesis.
An open configuration leads to a disrupted reaction pathway where critical intermediates are lost to the atmosphere.
This results in a negligible yield and a final product with poor crystallinity and defects in the graphitic sheets.
Making the Right Choice for Your Goal
To ensure reproducibility and quality in your material synthesis, apply the following guidelines:
- If your primary focus is High Yield: Ensure the crucible lid fits snugly to minimize mass loss from the sublimation of precursors like melamine or urea.
- If your primary focus is Material Quality: Use the semi-closed configuration to maintain the partial pressure required for forming a complete, defect-free two-dimensional layered structure.
Control the atmosphere, and you control the chemistry.
Summary Table:
| Feature | Open Crucible | Semi-Closed Crucible |
|---|---|---|
| Precursor Retention | Poor (High sublimation loss) | Excellent (Traps volatile vapors) |
| Micro-environment | Uncontrolled atmosphere | Self-pressurized reaction zone |
| Reaction Yield | Low to negligible | High (Maximum mass retention) |
| Structural Quality | Poor crystallinity/Defects | Uniform 2D layered structure |
| Safety/Pressure | No pressure risk | Managed venting of ammonia gas |
Optimize Your g-C3N4 Synthesis with KINTEK
Precise atmospheric control is the secret to high-yield material synthesis. KINTEK provides high-performance alumina crucibles and laboratory high-temp furnaces designed to maintain the exact thermal polycondensation environments your research demands.
Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable for your unique laboratory needs.
Ready to achieve superior material integrity? Contact KINTEK today to find the perfect specialized equipment for your application!
Visual Guide
References
- Yu‐Yun Lin, Chiing‐Chang Chen. Visible-Light-Driven Photocatalysis of Carbon Dioxide and Organic Pollutants by CaBiO2Cl/g-C3N4. DOI: 10.3390/molecules30183760
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- What is the function of the nitrogen environment in pyrolysis? Mastering Carbonization with Laboratory Furnaces
- Why is a glassy carbon boat preferred over an alumina crucible for Na3Cu4Se4? Ensuring Phase Purity in Flux Synthesis
- What mechanical properties should be evaluated for alumina ceramic furnace tubes? Ensure Durability and Performance
- What is the maximum vacuum capacity of the water circulating vacuum pump? Uncover Its Ideal Lab Applications
- What are the advantages of using a laboratory vacuum drying oven for modified ZnO nanomaterials? Protect Nano-Integrity
- What type of pump is used in water circulating vacuum pumps and how is it installed? Discover Robust Fluid-Based Vacuum Solutions
- What is the primary function of a high-energy planetary ball mill? Unlock Nanoscale Ceramic Pretreatment
- What is the maximum pressure achievable by the circulating water vacuum pump? Discover Its Vacuum Limits



















