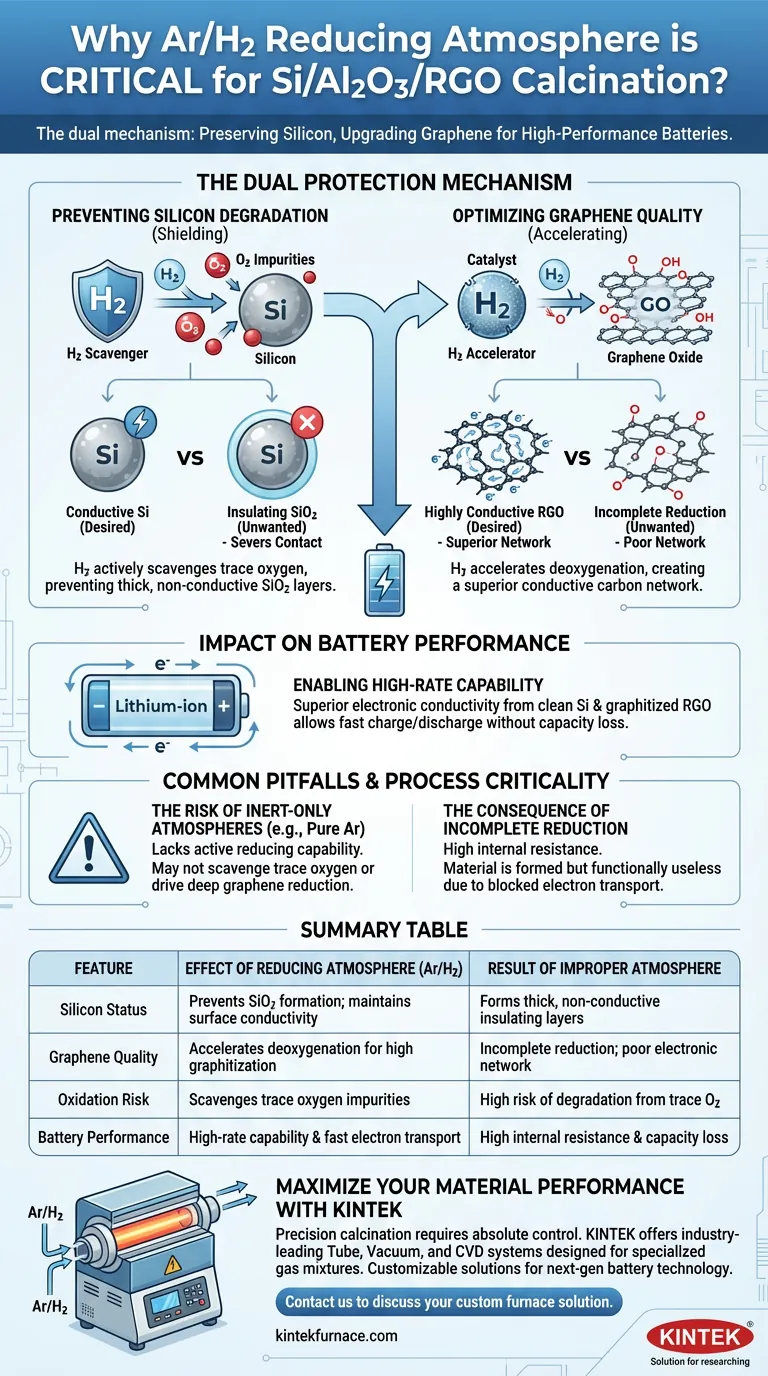
A reducing atmosphere is essential to preserve the electrical integrity of the composite material during calcination. Specifically, the Argon/Hydrogen (Ar/H2) mixture prevents the silicon component from degrading into an insulator while simultaneously upgrading the graphene oxide into a highly conductive network.
The Ar/H2 mixture serves a dual critical function: it acts as a chemical scavenger to stop silicon oxidation and as an accelerating agent for graphene reduction. Without this specific atmosphere, the material loses the electronic conductivity required for high-performance battery applications.
The Dual Protection Mechanism
Preventing Silicon Degradation
Silicon is highly susceptible to oxidation, even when only trace amounts of oxygen are present.
Without a reducing agent like Hydrogen, oxygen impurities in the furnace or the precursor materials react with the silicon particles.
This reaction forms thick, non-conductive silicon dioxide (SiO2) layers on the particle surface, which sever the electrical contact points necessary for the material to function as an anode.
Optimizing Graphene Quality
The atmosphere plays an active role in transforming Graphene Oxide (GO) into Reduced Graphene Oxide (RGO).
The presence of hydrogen gas accelerates the deoxygenation process, effectively stripping oxygen functional groups from the graphene lattice.
This results in a higher degree of graphitization, creating a superior conductive carbon network that wraps around and supports the silicon particles.
Impact on Battery Performance
Enabling High-Rate Capability
The primary goal of this composite is to function effectively in lithium-ion batteries, particularly under high current densities.
By preventing insulating SiO2 layers and ensuring the RGO is highly graphitized, the reducing atmosphere guarantees superior electronic conductivity.
This conductivity is the foundational requirement for improving rate performance, allowing the battery to charge and discharge quickly without significant capacity loss.
Common Pitfalls and Process Criticality
The Risk of Inert-Only Atmospheres
While inert gases like pure Argon are used in other processes (such as LFP synthesis) to prevent oxidation, they lack the active reducing capability of the Ar/H2 mix.
In the specific context of Si/Al2O3/RGO, a purely inert atmosphere may not be sufficient to scavenge trace oxygen or drive the deep reduction of graphene oxide.
The Consequence of Incomplete Reduction
If the atmosphere is not sufficiently reducing, the resulting composite will suffer from high internal resistance.
This leads to a material that is technically "formed" but functionally useless for high-performance applications due to the blocking of electron transport pathways.
Making the Right Choice for Your Goal
To ensure the successful synthesis of Si/Al2O3/RGO composites, consider the following regarding your furnace atmosphere:
- If your primary focus is preserving Silicon capacity: Ensure the H2 concentration is sufficient to scavenge all trace oxygen, preventing the formation of insulating SiO2 barriers.
- If your primary focus is maximizing Rate Performance: Prioritize the reducing atmosphere to achieve the highest possible degree of graphitization in the RGO network for rapid electron transfer.
The specific chemistry of the Ar/H2 atmosphere is not just a protective measure; it is an active participant in defining the final electrochemical power of your material.
Summary Table:
| Feature | Effect of Reducing Atmosphere (Ar/H2) | Result of Improper Atmosphere |
|---|---|---|
| Silicon Status | Prevents SiO2 formation; maintains surface conductivity | Forms thick, non-conductive insulating layers |
| Graphene Quality | Accelerates deoxygenation for high graphitization | Incomplete reduction; poor electronic network |
| Oxidation Risk | Scavenges trace oxygen impurities | High risk of degradation from trace O2 |
| Battery Performance | High-rate capability & fast electron transport | High internal resistance & capacity loss |
Maximize Your Material Performance with KINTEK
Precision calcination of advanced composites like Si/Al2O3/RGO requires absolute control over atmospheric conditions. KINTEK provides industry-leading Tube, Vacuum, and CVD systems designed to handle specialized gas mixtures like Ar/H2 with unmatched stability.
Backed by expert R&D and manufacturing, our high-temperature furnaces are fully customizable to meet your unique research or production needs—ensuring your materials achieve the graphitization and conductivity required for next-gen battery technology.
Ready to optimize your synthesis process? Contact us today to discuss your custom furnace solution with our technical team.
Visual Guide
References
- Xiangyu Tan, Xin Cai. Reduced graphene oxide-encaged submicron-silicon anode interfacially stabilized by Al<sub>2</sub>O<sub>3</sub> nanoparticles for efficient lithium-ion batteries. DOI: 10.1039/d4ra00751d
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
People Also Ask
- What is the role of a laboratory high-temperature annealing furnace in preparing amorphous (InxGa1-x)2O3 thin films?
- What is the significance of the controlled oxygen partial pressure in REBCO superconducting tapes oxygenation?
- What is the function of a laboratory vacuum drying oven in the treatment of catalyst powders? | Expert Guide
- What is the mechanism by which a reducing atmosphere improves Mn-Zn ferrite performance? Unlocking Magnetic Excellence
- What related term is mentioned in connection with controlled atmosphere furnaces? Discover Sealed Quench for Superior Heat Treatment
- What safety features are included in the box type annealing atmosphere furnace? Ensure Operator and Equipment Protection
- What are the two main purposes of furnace atmospheres? Achieve Superior Material Protection and Surface Engineering
- What types of configurations are available for retort furnaces? Optimize Your Thermal Process with the Right Setup



















