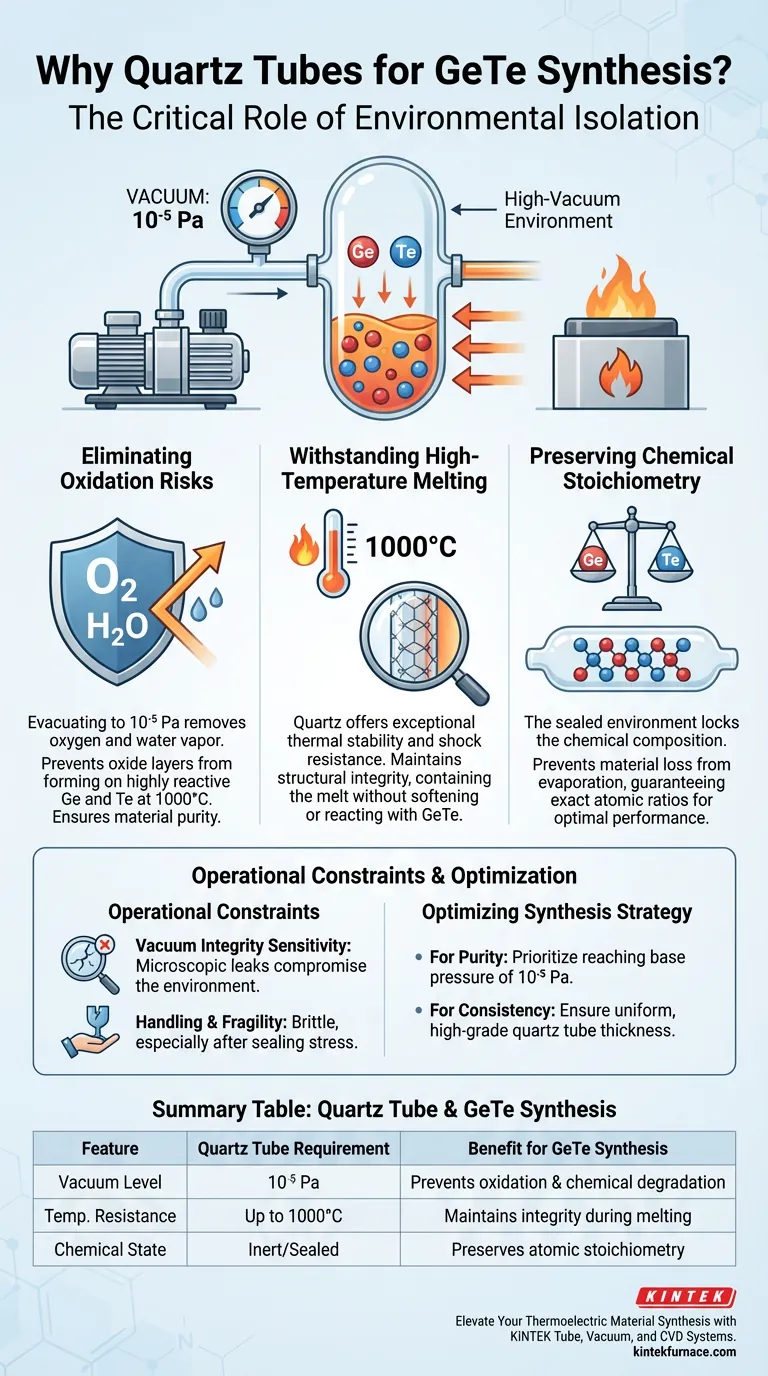
The primary reason for using a quartz tube is to create an absolutely inert environment that isolates reactive elements from atmospheric oxygen. During the synthesis of GeTe-based materials, the tube is evacuated to a high vacuum (typically $10^{-5}$ Pa) and sealed to prevent oxidation. This step is non-negotiable because germanium and tellurium are highly susceptible to chemical degradation when subjected to the necessary melting temperatures of $1000^\circ\text{C}$.
Core Takeaway: By creating a sealed, high-vacuum environment, the quartz tube acts as a barrier against oxidation and impurities. This isolation ensures that the final thermoelectric material maintains the precise chemical stoichiometry required for optimal semiconductor performance.

The Critical Role of Environmental Isolation
Eliminating Oxidation Risks
The synthesis of Germanium Telluride (GeTe) involves heating raw elements to extreme temperatures. Under normal atmospheric conditions, both germanium and tellurium react aggressively with oxygen at these heat levels.
By evacuating the quartz tube to a pressure of $10^{-5}$ Pa, you effectively remove oxygen and water vapor. This prevents the formation of oxide layers that would otherwise degrade the purity and performance of the thermoelectric material.
Withstanding High-Temperature Melting
The synthesis process requires temperatures reaching $1000^\circ\text{C}$ to properly melt and alloy the components. Quartz is selected because it possesses exceptional thermal stability and thermal shock resistance.
Unlike standard glass or many metals, quartz maintains its structural integrity at these temperatures. It provides a reliable physical barrier that contains the melt without softening or chemically reacting with the GeTe compound.
Preserving Chemical Stoichiometry
Thermoelectric performance relies heavily on an exact atomic ratio (stoichiometry) between the elements. Any loss of material due to reaction with air or evaporation would alter this delicate balance.
The sealed quartz environment locks the chemical composition in place. It ensures that the ratio of reactants you weigh at the start matches the composition of the final crystal, guaranteeing the intended electronic properties.
Understanding the Operational Constraints
Vacuum Integrity Sensitivity
The efficacy of this method relies entirely on the quality of the vacuum seal. Even a microscopic leak or a failure to reach the $10^{-5}$ Pa threshold will introduce enough oxygen to compromise the material.
Handling and Fragility
While thermally robust, quartz is mechanically brittle. The sealing process involves melting the quartz neck under vacuum, which introduces stress points that can fracture if handled improperly during the cooling or quenching phases.
Optimizing Your Synthesis Strategy
To ensure high-performance GeTe material production, apply the following guidelines based on your specific objectives:
- If your primary focus is material purity: Prioritize reaching a base pressure of at least $10^{-5}$ Pa before sealing to eliminate all traces of atmospheric contaminants.
- If your primary focus is process consistency: Ensure the quartz tube wall thickness is uniform and high-grade to withstand the $1000^\circ\text{C}$ thermal load without deformation.
The quartz tube is not merely a container; it is the fundamental control mechanism for chemical precision in high-temperature synthesis.
Summary Table:
| Feature | Quartz Tube Requirement | Benefit for GeTe Synthesis |
|---|---|---|
| Vacuum Level | $10^{-5}$ Pa | Prevents oxidation and chemical degradation |
| Temp. Resistance | Up to $1000^\circ\text{C}$ | Maintains structural integrity during melting |
| Chemical State | Inert/Sealed | Preserves precise atomic stoichiometry |
| Thermal Property | High Shock Resistance | Prevents fracturing during rapid quenching |
Elevate Your Thermoelectric Material Synthesis with KINTEK
Precise temperature control and vacuum integrity are critical for high-performance GeTe production. KINTEK provides industry-leading Tube, Vacuum, and CVD systems designed to meet the rigorous demands of advanced material science. Backed by expert R&D and manufacturing, our lab high-temp furnaces are fully customizable to your unique research needs.
Ready to optimize your synthesis process? Contact us today to discover how KINTEK’s specialized heating solutions can ensure the purity and consistency of your materials.
Visual Guide
References
- Tao Guo, Lingling Ren. Study on the Effect of Sn, In, and Se Co-Doping on the Thermoelectric Properties of GeTe. DOI: 10.3390/ma17030551
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
People Also Ask
- How does the quartz tube furnace minimize heat loss? Double Insulation for Energy Efficiency
- How to clean a tubular furnace? A Step-by-Step Guide to Safe and Effective Maintenance
- What is the Purpose of Carbon Coating Quartz Tubes? Enhance Crystal Growth via Bridgman Method
- Why is precise temperature control in a tubular furnace essential for SiO2/C microspheres? Master Carbonization Success
- What is the core role of a tube furnace in synthesizing magnetic carbon-based composites? Expert Insights
- How do horizontal furnaces contribute to cost savings in industrial processes? Boost Efficiency & Cut Costs
- How is the atmosphere controlled in a vacuum tube furnace? Achieve Precise Gas Environments for Your Experiments
- What is a tubular furnace and what are its primary uses? Essential for High-Temperature Precision and Uniformity



















