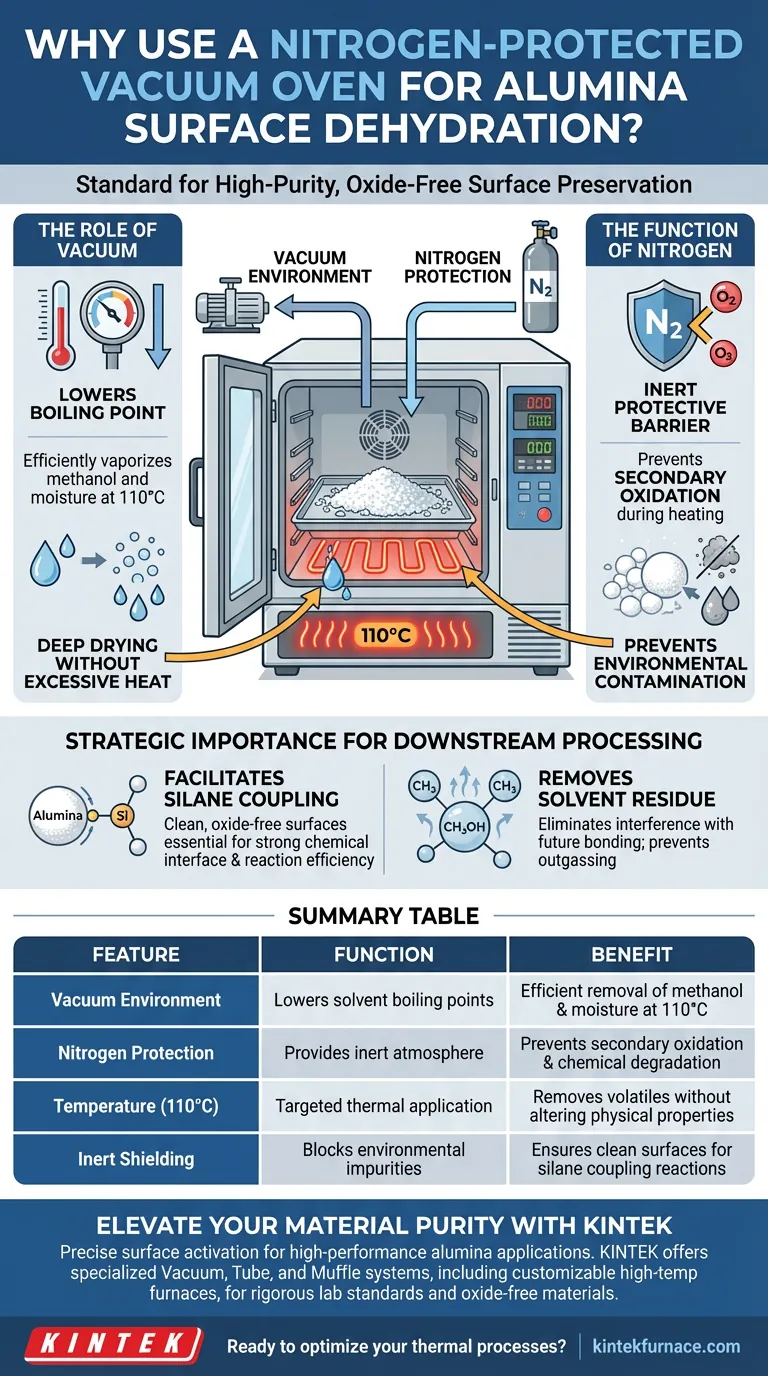
A laboratory vacuum oven with nitrogen protection is the standard for achieving high-purity dehydration of alumina surfaces. This specialized process utilizes a temperature of 110°C in a vacuum to strip away residual methanol solvents and moisture, while the nitrogen atmosphere actively shields the particles from chemical degradation.
Core Insight: The primary goal of this setup is not just drying, but surface preservation. By replacing reactive air with inert nitrogen under vacuum, you eliminate contaminants without risking secondary oxidation, ensuring the alumina is chemically primed for subsequent reactions.
The Mechanisms of Protection and Purification
The Role of the Vacuum Environment
The vacuum aspect of the oven serves a thermodynamic purpose: it significantly lowers the boiling point of solvents.
By reducing the pressure, residual methanol and adsorbed moisture can vaporize efficiently at 110°C. This ensures deep drying without requiring excessive heat that might otherwise alter the material's physical properties.
The Function of Nitrogen Gas
Heat accelerates chemical reactions, including unwanted ones. Nitrogen is introduced as an inert protective barrier.
During the drying process, the nitrogen atmosphere prevents oxygen from interacting with the heated alumina. This eliminates the risk of secondary oxidation, keeping the surface chemistry stable.
Preventing Environmental Contamination
Standard drying methods often expose materials to airborne particulates or humidity.
The nitrogen-filled vacuum chamber prevents the adsorption of environmental impurities. This isolation is critical for maintaining the strict cleanliness standards required for high-performance ceramic applications.
Strategic Importance for Downstream Processing
Facilitating Silane Coupling
The ultimate objective of this rigorous drying process is often to prepare the alumina for surface modification.
Clean, oxide-free surfaces are essential for facilitating reactions with silane coupling agents. If the surface is contaminated or oxidized, the coupling agents cannot bond effectively, leading to poor material performance.
Removing Solvent Residue
The process is specifically tuned to remove methanol, a common solvent used in earlier processing stages.
Residual solvents can interfere with future chemical bonding or outgas during later use. The 110°C vacuum environment ensures these volatile components are completely evacuated from the particle surface.
Understanding the Process Requirements
Equipment Dependencies
This method requires more than a standard drying oven; it demands a sealed system capable of maintaining a vacuum and a regulated nitrogen supply.
The integrity of the seal is paramount. Any leak introduces oxygen or moisture, negating the protective benefits of the nitrogen and potentially ruining the surface activation.
Thermal Precision
The temperature of 110°C is specific. It is high enough to drive off the targeted volatiles (methanol and water) under vacuum but controlled enough to prevent sintering or thermal shock to the powder.
Making the Right Choice for Your Goal
To determine if this rigorous drying method is necessary for your application, evaluate your downstream chemistry requirements.
- If your primary focus is Silane Bonding: This process is mandatory to ensure the surface cleanliness required for a strong chemical interface.
- If your primary focus is Bulk Drying: A standard oven may suffice, but you risk surface oxidation and impurity adsorption.
Summary: Use nitrogen-protected vacuum drying when the chemical purity of the alumina surface is a non-negotiable factor for reaction efficiency.
Summary Table:
| Feature | Function in Alumina Dehydration | Benefit for Materials |
|---|---|---|
| Vacuum Environment | Lowers solvent boiling points | Efficient removal of methanol and moisture at 110°C |
| Nitrogen Protection | Provides an inert atmosphere | Prevents secondary oxidation and chemical degradation |
| Temperature (110°C) | Targeted thermal application | Removes volatiles without altering physical properties |
| Inert Shielding | Blocks environmental impurities | Ensures clean surfaces for silane coupling reactions |
Elevate Your Material Purity with KINTEK
Precise surface activation is the key to high-performance alumina applications. Backed by expert R&D and world-class manufacturing, KINTEK offers specialized Vacuum, Tube, and Muffle systems designed to meet the most rigorous lab standards. Whether you need standard dehydration or a fully customizable high-temp furnace tailored to your unique chemical requirements, our equipment ensures your materials remain oxide-free and ready for downstream processing.
Ready to optimize your thermal processes? Contact KINTEK today to find your solution.
Visual Guide
References
- Seul-Ki Kim, Eun Young Jung. Fabrication and Characterization of Al2O3-Siloxane Composite Thermal Pads for Thermal Interface Materials. DOI: 10.3390/ma17040914
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- How does a vacuum furnace improve the mechanical properties of workpieces? Enhance Strength and Durability
- What benefits does a vacuum quenching furnace provide in tool manufacturing? Achieve Superior Hardness and Precision
- How does a laboratory vacuum high-temperature furnace maintain conditions for LiF-BeF2-LaF3? Expert Atmosphere Control
- Why is a vacuum oven required for FeZn-MOFs@Al2O3? Preserve Structure and Activity
- What functions does a high-density graphite mold serve in the SPS process? Beyond Shaping Materials
- What are the steps involved in the vacuum sintering process? Master Precision and Purity for Superior Materials
- What operational advantages do vacuum furnaces provide? Achieve Superior Material Quality and Process Control
- What industries commonly use vacuum brazing furnaces? Essential for Aerospace, Medical, Automotive, and Electronics



















