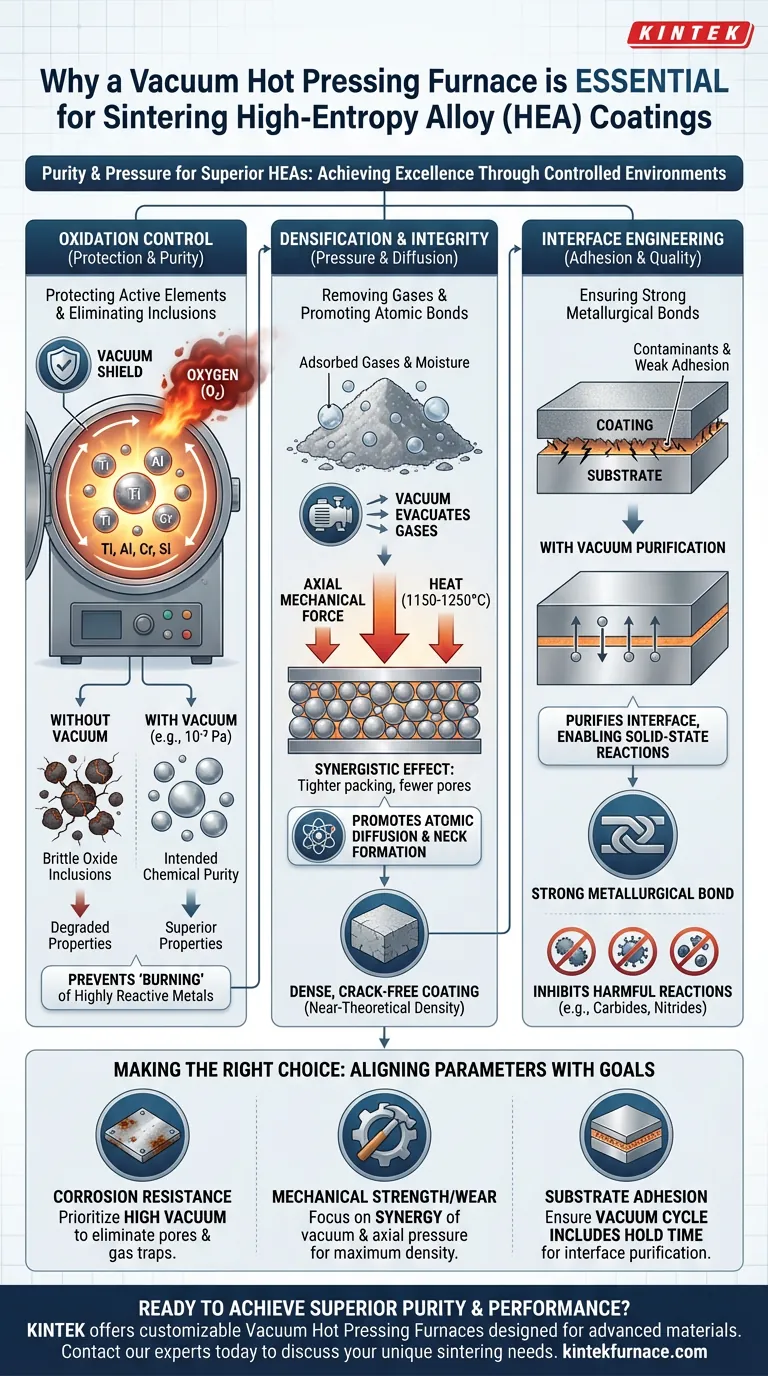
A hot pressing furnace equipped with a vacuum system is essential for sintering high-entropy alloy (HEA) coatings because it creates a pristine environment that prevents chemical degradation while applying mechanical force. By significantly lowering the oxygen partial pressure, the system eliminates the risk of oxidation and removes trapped gases, allowing chemically active elements to bond metallurgically rather than reacting with atmospheric impurities.
Core Takeaway Achieving the superior mechanical properties of high-entropy alloys requires more than just heat; it requires absolute chemical purity. The vacuum hot pressing furnace acts as a dual-force chamber, applying thermal and mechanical energy to promote atomic diffusion while simultaneously stripping away contaminants to ensure a dense, crack-free, and corrosion-resistant material.

The Critical Role of Oxidation Control
Protecting Highly Active Elements
High-entropy alloys often contain chemically active elements such as Titanium (Ti), Aluminum (Al), Chromium (Cr), and Silicon (Si). These metals are highly reactive with oxygen, especially at the elevated temperatures required for sintering (often between 1150°C and 1250°C).
Without a vacuum, these elements would rapidly oxidize. A vacuum system effectively isolates the material from air, preventing the "burning" or oxidation of these powders during the heating process.
Eliminating Oxide Inclusions
The presence of oxygen creates brittle oxide inclusions within the alloy matrix. These inclusions act as stress concentration points, which can severely degrade the mechanical properties of the coating.
By maintaining a high vacuum (e.g., 10⁻³ Pa or better), the furnace prevents these oxides from forming. This ensures the final coating retains the intended chemical purity and avoids the embrittlement often caused by excessive oxygen content.
Densification and Structural Integrity
Removing Adsorbed Gases
Metal powders naturally adsorb gases and moisture on their surfaces and within inter-particle gaps. If these gases are not removed during sintering, they become trapped, resulting in a porous, low-density coating.
The vacuum environment actively evacuates gases from powder interstices and surfaces. This allows for tighter packing of the powder particles, which is a prerequisite for achieving near-theoretical density in the final sintered body.
Promoting Atomic Diffusion
Sintering relies on the diffusion of atoms across particle boundaries to form "necks." The vacuum hot pressing furnace utilizes a synergistic effect of heat and axial force.
The vacuum purifies the particle interfaces, removing barriers to diffusion. Simultaneously, the mechanical pressure drives the particles together. This combination promotes rapid densification, resulting in a coating that is free of cracks and pores.
Interface Engineering and Bonding
Ensuring Metallurgical Bonding
For a coating to be effective, it must adhere perfectly to the substrate. Contaminants at the interface can lead to delamination or weak adhesion.
The vacuum environment purifies the material interface, ensuring effective solid-state reactions between elements. This facilitates the formation of a strong metallurgical bond between the coating and the substrate, significantly improving interfacial quality.
Inhibiting Harmful Reactions
In certain alloy systems, the presence of air can lead to nitridation or the formation of unwanted carbides (such as aluminum carbide in specific matrices). These interfacial reaction products can be detrimental to the material's performance.
A controlled vacuum atmosphere inhibits the excessive formation of these harmful phases. This control is decisive for improving both the material density and the quality of the interfacial bond.
Understanding the Trade-offs
The Risk of Insufficient Vacuum
While vacuum systems are powerful, they require precise control. If the vacuum level is insufficient (e.g., not reaching 10⁻³ Pa for highly sensitive alloys like Ti-Al-Nb), the protective benefits are lost.
Partial oxidation can still occur, leading to material embrittlement. Even a small amount of residual oxygen can compromise the ductility and fatigue strength of the alloy, rendering the high-entropy design useless.
Operational Complexity
Vacuum hot pressing is inherently more complex than atmospheric sintering. It requires managing not just temperature and pressure, but also maintaining a rigorous seal and specific pressure thresholds (such as 0.133 Pa for Cr-Si systems).
This adds variables to the manufacturing process. A failure in the vacuum seal or pump system during the heating cycle can result in the total loss of the batch due to rapid oxidation of the powder surface.
Making the Right Choice for Your Goal
To maximize the performance of your high-entropy alloy coatings, align your sintering parameters with your specific performance targets:
- If your primary focus is Corrosion Resistance: Prioritize high vacuum levels to eliminate pores and adsorbed gases, as these defects act as initiation sites for corrosion.
- If your primary focus is Mechanical Strength/Wear: Focus on the synergy of vacuum and axial pressure to ensure maximum density and the elimination of brittle oxide inclusions.
- If your primary focus is Substrate Adhesion: Ensure the vacuum cycle includes a hold time that allows for thorough purification of the interface to guarantee metallurgical bonding.
The vacuum hot pressing furnace is not merely a heater; it is a purification tool that enables the atomic-level engineering required for high-performance alloy coatings.
Summary Table:
| Key Benefit | Role of Vacuum Hot Pressing |
|---|---|
| Oxidation Control | Prevents reaction of active elements (Ti, Al, Cr) with oxygen, eliminating brittle oxide inclusions. |
| Densification | Evacuates trapped gases from powder, promoting atomic diffusion and near-theoretical density under pressure. |
| Interface Bonding | Purifies the coating-substrate interface, enabling strong metallurgical bonds and preventing delamination. |
| Performance Target | Recommended Focus |
| Corrosion Resistance | Prioritize high vacuum levels to eliminate pores and gas traps. |
| Mechanical Strength/Wear | Focus on synergy of vacuum and axial pressure for maximum density. |
| Substrate Adhesion | Ensure vacuum cycle includes hold time for interface purification. |
Ready to achieve the superior purity and performance of your high-entropy alloy coatings?
Backed by expert R&D and manufacturing, KINTEK offers customizable Muffle, Tube, Rotary, Vacuum, and CVD furnace systems, including specialized vacuum hot pressing furnaces designed for the precise demands of advanced materials like HEAs. Our solutions ensure the critical control over oxidation, densification, and bonding your research and production require.
Contact our experts today to discuss how we can tailor a furnace system to your unique sintering needs and performance goals.
Visual Guide
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- What are the key steps in vacuum hot pressing? Achieve High-Density Materials for Demanding Applications
- Why is a vacuum essential for TiAl/Ti6Al4V hot pressing? Unlock High-Performance Metallurgical Bonding
- How does a hot press machine work? Master Heat, Pressure, and Time for Perfect Results
- What is the role of the vacuum environment in SiC/ZTA sintering? Enhance Densification & Material Purity
- What control features does a vacuum hot press furnace offer? Precision Control for Advanced Materials Processing
- What is a vacuum press used for? Achieve Flawless Bonding and Material Transformation
- What core role does a vacuum hot press furnace play in the densification process of copper-carbon nanotube composites? Achieve High-Performance Cu-CNT Materials
- What is the core function of a vacuum hot press sintering furnace in the preparation of high-density RuTi alloys? Achieve Maximum Density and Purity



















