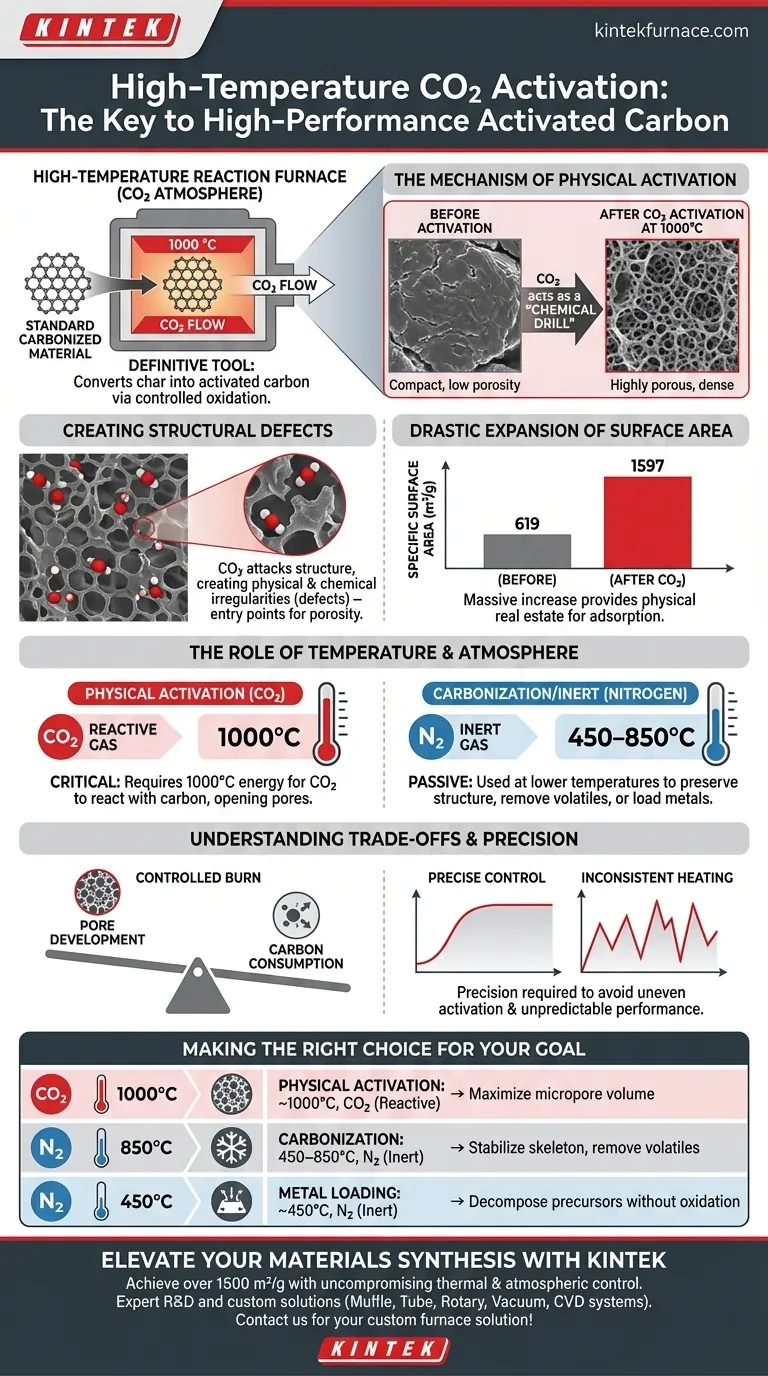
A high-temperature reaction furnace with carbon dioxide control is the definitive tool for converting standard carbonized material into high-performance activated carbon. By introducing CO2 at approximately 1000 °C, the furnace initiates a controlled oxidation process that physically and chemically alters the carbon matrix. This treatment creates essential structural defects, drastically expanding micropore volume to enhance adsorption capabilities.
The specific combination of extreme heat and a carbon dioxide atmosphere acts as a chemical drill. It transforms a low-surface-area skeleton into a highly porous structure, unlocking the physical space required to capture contaminants like mercury.

The Mechanism of Physical Activation
Creating Structural Defects
The introduction of carbon dioxide acts as an activating agent rather than a passive gas. It attacks the carbon structure, creating physical and chemical irregularities known as structural defects.
These defects are not flaws; they are the entry points for porosity. This "etching" process is what differentiates simple charred carbon from true activated carbon.
Drastic Expansion of Surface Area
The impact of this process on the material's physical properties is profound. The treatment significantly increases both the specific surface area and the micropore volume.
For example, data indicates that CO2 activation at 1000 °C can raise the specific surface area from 619 m²/g to 1597 m²/g. This massive increase provides the necessary physical real estate for adsorption applications, such as mercury removal.
The Role of Temperature and Atmosphere
Why 1000 °C is Critical
High temperatures are non-negotiable for this type of physical activation. While lower temperatures (around 850 °C) are sufficient for carbonization in nitrogen, CO2 activation requires the energy of 1000 °C to drive the reaction.
At this thermal tier, the thermodynamic conditions allow the CO2 to react with the carbon skeleton effectively. Without this extreme heat, the activation energy barrier would not be overcome, and the pore structure would remain undeveloped.
Comparison to Inert Atmospheres
It is vital to distinguish this process from inert treatments. An inert atmosphere (like Nitrogen) is typically used at lower temperatures (around 450–850 °C) to prevent oxidation or remove volatiles.
In contrast, the CO2 atmosphere is intentionally reactive. It is designed to consume parts of the carbon to open up pores, whereas nitrogen is designed to preserve the existing structure.
Understanding the Trade-offs
Carbon Consumption vs. Pore Development
The process of activation is essentially a controlled burn. To create pores, you must sacrifice a portion of the carbon matrix.
If the furnace temperature fluctuates or the exposure time is too long, you risk "over-activation," where the pore walls collapse and the material yield drops significantly.
Precision Requirements
Because of the delicate balance between creating pores and destroying the material, the furnace must offer high-precision temperature control. Inconsistent heating can lead to uneven activation, resulting in a batch of material with unpredictable adsorption performance.
Making the Right Choice for Your Goal
To achieve the correct material properties, you must match the furnace atmosphere and temperature to your specific processing stage.
- If your primary focus is increasing surface area: You must use a CO2 atmosphere at approximately 1000 °C to etch the carbon matrix and maximize micropore volume.
- If your primary focus is stabilizing the carbon skeleton: You should use an inert Nitrogen atmosphere at roughly 850 °C to remove volatiles without consuming the carbon.
- If your primary focus is loading active metals (e.g., Copper): You should use a lower-temperature Nitrogen flow (around 450 °C) to decompose precursors without oxidizing the carbon support.
The precise control of atmosphere and heat determines whether you produce a simple char or a high-capacity adsorbent.
Summary Table:
| Activation Parameter | Atmosphere | Temperature Range | Primary Effect on Material |
|---|---|---|---|
| Physical Activation | CO2 (Reactive) | ~1000 °C | Creates structural defects; increases surface area (e.g., 619 to 1597 m²/g). |
| Carbonization | Nitrogen (Inert) | 450 – 850 °C | Removes volatiles; stabilizes carbon skeleton without oxidation. |
| Metal Loading | Nitrogen (Inert) | ~450 °C | Decomposes precursors (e.g., Copper) without damaging carbon support. |
Elevate Your Materials Synthesis with KINTEK
Precision is the difference between simple char and high-capacity activated carbon. At KINTEK, we understand that achieving a specific surface area of over 1500 m²/g requires uncompromising thermal and atmospheric control.
Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet your unique high-temperature and reactive gas requirements. Whether you are scaling up CO2 activation at 1000 °C or performing delicate precursor decomposition, our lab high-temp furnaces provide the stability and precision your research demands.
Ready to optimize your activation process? Contact us today to find your custom furnace solution!
Visual Guide
References
- M. Antonia López-Antón, Ana Arenillas. Mercury Removal by Carbon Materials with Emphasis on the SO <sub>2</sub> –Porosity Relationship. DOI: 10.1002/open.202500190
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- What is the purpose of an atmosphere furnace? Control Gas Environments for Superior Material Processing
- What is the function of a high-pressure Argon atmosphere? Master Complex Alloy Purity with Precision Melting
- What is the purpose of the secondary heat treatment in an annealing furnace? Enhance S@Se-ZnS/HSC Material Stability
- What are the key features of a retort furnace? Unlock Precise Atmospheric Control for Advanced Processes
- What is the purpose of introducing a nitrogen protective atmosphere during the continuous annealing of silicon steel?
- What is the function of a high-temperature calcination furnace? Mastering Pr3+:CaGdF2 Nanopowder Precursor Prep
- What are the five key components of atmosphere furnaces? Master Controlled Heat Treatment for Superior Results
- Why is a carbothermic reduction step necessary for copper slag glass-ceramics? Optimize Your Material Purification



















