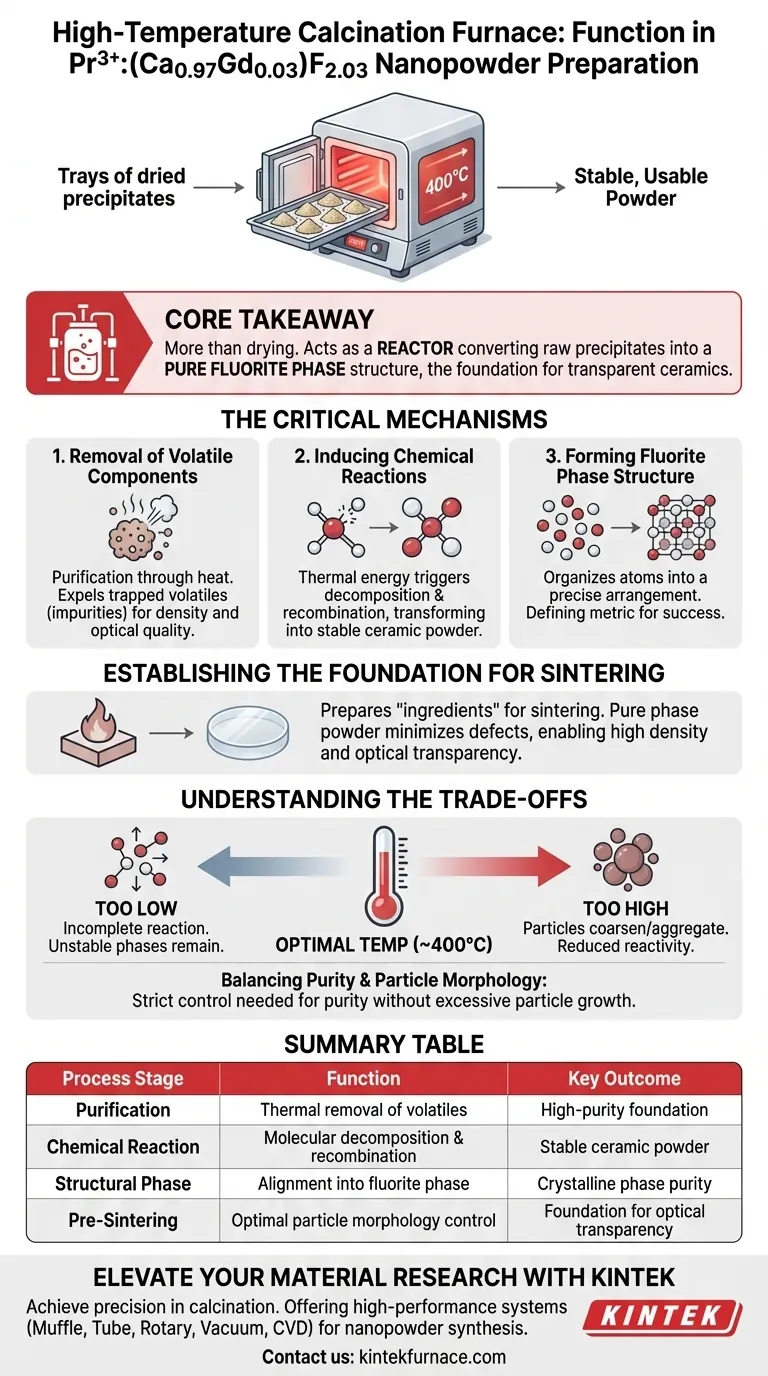
In the preparation of Pr3+:(Ca0.97Gd0.03)F2.03 nanopowder precursors, a high-temperature calcination furnace performs the essential function of heat-treating dried precipitates at precise temperatures, typically around 400°C. This thermal treatment is the pivotal step that converts raw chemical precipitates into a stable, usable powder by removing volatile byproducts and driving the chemical changes necessary to form the final material structure.
Core Takeaway The calcination furnace does not merely dry the material; it acts as a reactor that strips away volatile impurities and chemically rearranges the precursor into a pure fluorite phase structure, establishing the mandatory foundation for creating transparent ceramics later in the process.

The Critical Mechanisms of Calcination
Removal of Volatile Components
The initial role of the furnace is purification through heat. As the temperature rises, volatile components trapped within the dried precursor precipitates are expelled.
Eliminating these volatiles is non-negotiable. If left in the material, these impurities would disrupt the density and optical quality of the final product.
Inducing Chemical Reactions
Beyond purification, the furnace provides the thermal energy required to trigger specific chemical reactions. The heat forces the precursor materials to decompose and recombine at the molecular level.
This transition transforms the chemically active precipitate into a stable ceramic powder.
Forming the Fluorite Phase Structure
The ultimate goal of this heat treatment is structural organization. The process guides the atoms into a precise arrangement known as a pure fluorite phase structure.
Achieving this specific crystalline phase is the defining success metric of the calcination step. Without this phase purity, the material cannot perform its function in optical applications.
Establishing the Foundation for Sintering
Preparing for Transparency
The calcination furnace essentially prepares the "ingredients" for the final "baking" process (sintering). By creating a pure phase powder, the furnace ensures the material is ready to be compressed and heated into a solid body.
Preventing Structural Defects
The phase foundation established here dictates the quality of the final ceramic. If the powder is correctly calcined, it minimizes the risk of defects during the subsequent sintering stage.
A well-calcined powder is the prerequisite for achieving high density and optical transparency in the final ceramic component.
Understanding the Trade-offs
The Risk of Incorrect Temperature
Precision is paramount; the furnace must maintain specific temperatures (e.g., 400°C) to ensure the reaction is complete without damaging the material.
If the temperature is too low, the chemical reaction may remain incomplete, leaving unstable phases in the powder. If the temperature is too high, particles may coarsen or aggregate, reducing their reactivity for the next stage.
Balancing Purity and Particle Morphology
While the primary goal is phase purity, the calcination process also impacts particle characteristics.
Strict control is required to remove impurities without causing the particles to grow too large, as fine particle size is often preferred for high-performance ceramics.
Making the Right Choice for Your Goal
To ensure the highest quality Pr3+:(Ca0.97Gd0.03)F2.03 nanopowders, consider the following based on your specific objectives:
- If your primary focus is Phase Purity: Ensure the furnace is capable of maintaining a stable 400°C to fully drive the conversion to the fluorite phase structure.
- If your primary focus is Final Optical Transparency: Prioritize the complete removal of volatile components during calcination to prevent pore formation during the subsequent sintering step.
The success of transparent ceramic sintering is determined before the sintering ever begins—it starts with precise control in the calcination furnace.
Summary Table:
| Process Stage | Function of Furnace | Key Outcome |
|---|---|---|
| Purification | Thermal removal of volatile byproducts | High-purity material foundation |
| Chemical Reaction | Molecular decomposition and recombination | Stable ceramic powder formation |
| Structural Phase | Alignment into pure fluorite phase | Crystalline phase purity |
| Pre-Sintering | Optimal particle morphology control | Foundation for optical transparency |
Elevate Your Material Research with KINTEK
Achieve uncompromising precision in your calcination processes. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems designed to meet the rigorous demands of nanopowder synthesis. Whether you need a standard solution or a customizable system for unique thermal profiles, our lab high-temp furnaces ensure the phase purity and stability your research deserves.
Ready to optimize your precursor preparation? Contact us today to find the perfect furnace solution!
Visual Guide
Related Products
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
People Also Ask
- What are the common gases and vapors used in furnace atmospheres and their roles? Optimize Your Heat Treatment Process
- Why would a heat treatment process require an inert atmosphere furnace? Prevent Oxidation and Ensure Material Integrity
- What is a reducing atmosphere? Master Material Protection and Control
- What types of gases are commonly used in atmosphere furnaces and what are their purposes? Optimize Your Heat Treatment Processes
- Why is a controlled atmosphere furnace required for 316L debinding? Ensure Structural Integrity & Zero Cracks
- Why is a high-temperature reaction furnace with CO2 control necessary for activated carbon? Unlock Maximum Porosity
- Why is a reducing atmosphere important? Prevent Oxidation for Superior Material Processing
- What is the role of a benchtop drying oven in the preparation of Cu/TiO2/ZSM-5 catalysts? Ensure Optimal Dispersion



















