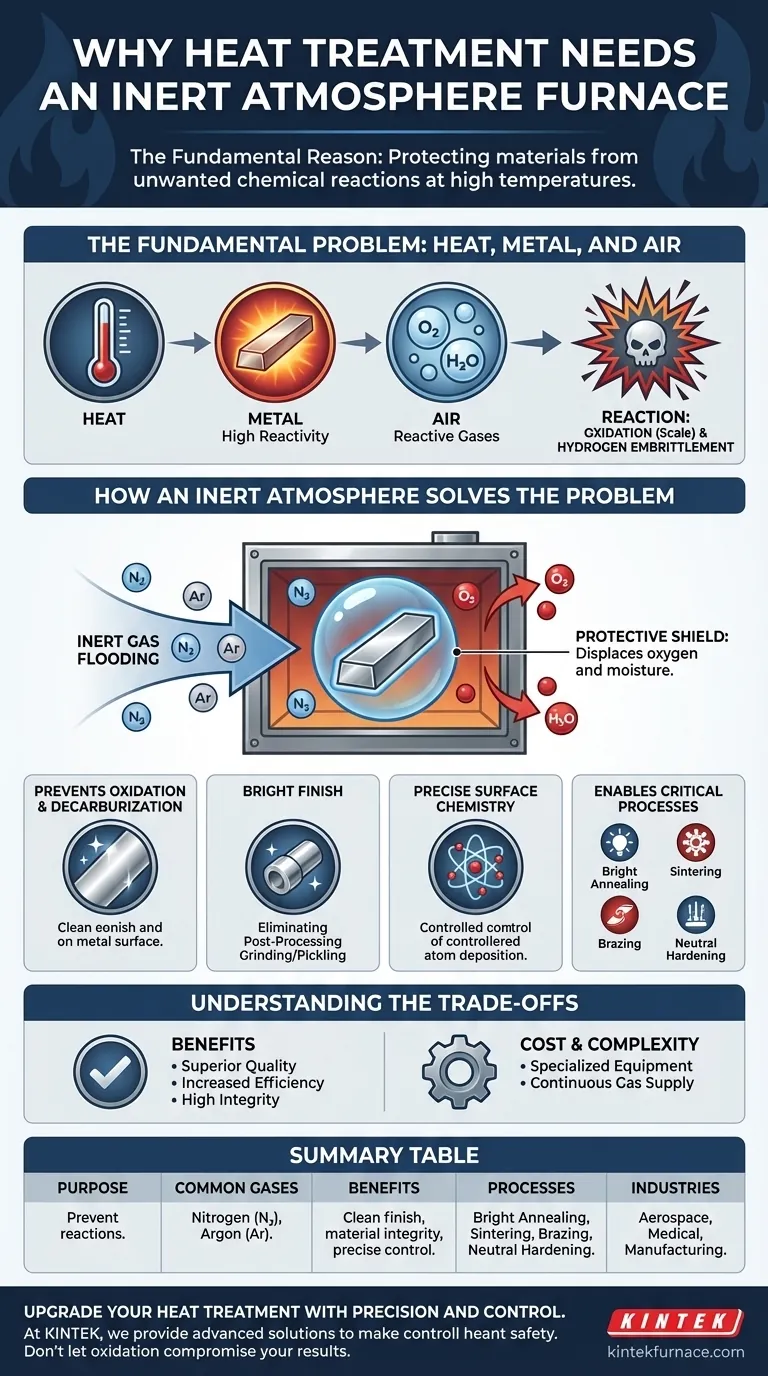
The fundamental reason a heat treatment process requires an inert atmosphere furnace is to protect the material from unwanted chemical reactions, primarily oxidation, at high temperatures. When metals are heated, they become highly reactive with gases in the air, such as oxygen and moisture. An inert atmosphere displaces these reactive gases, preventing surface damage like scaling and ensuring the material's final properties meet precise engineering specifications.
An inert atmosphere is not merely about preventing rust. It is a strategic tool for controlling the surface chemistry of a component, ensuring its structural integrity, appearance, and performance are achieved without compromise.

The Fundamental Problem: Heat, Metal, and Air
Heat treatment relies on precise thermal cycles to alter a material's microstructure. However, the very heat that enables these positive changes also makes the material chemically vulnerable.
Why High Temperatures Are a Challenge
Heat acts as a catalyst for chemical reactions. As a metal's temperature rises, its atoms vibrate more energetically, making it significantly more susceptible to reacting with its surrounding environment.
The Role of Oxygen
The most common and damaging reaction at high temperatures is oxidation. Oxygen in the ambient air aggressively bonds with hot metal to form a layer of oxides, often called scale. This scale is brittle, alters the part's dimensions, and results in a discolored, rough surface finish.
Other Atmospheric Contaminants
Beyond oxygen, other elements in the air can cause issues. Moisture (H₂O) can introduce hydrogen into the material's structure, leading to a dangerous condition known as hydrogen embrittlement, which severely reduces ductility and toughness.
How an Inert Atmosphere Solves the Problem
An inert atmosphere furnace systematically replaces the reactive air inside the heating chamber with a non-reactive gas, effectively creating a protective bubble around the workpiece.
Creating a Protective Shield
Gases like nitrogen (N₂) and argon (Ar) are chemically inert, meaning they do not readily react with other elements, even at high temperatures. By flooding the furnace with one of these gases, oxygen and moisture are purged, starving the potential for unwanted chemical reactions.
Preventing Oxidation and Decarburization
The most immediate benefit is the complete prevention of oxidation. This results in a clean, scale-free surface, often referred to as a "bright" finish, which eliminates the need for costly and time-consuming secondary cleaning operations like grinding or acid pickling. For steels, it also prevents decarburization—the loss of carbon from the surface, which would otherwise soften the material.
Enabling Precise Surface Chemistry
In some processes, the atmosphere is not just protective but also an active ingredient. Processes like carbonitriding intentionally add carbon and nitrogen to a steel surface to harden it. An inert gas like nitrogen acts as a neutral carrier gas, delivering the active chemical components to the surface in precise concentrations without interference from oxygen.
Understanding the Trade-offs and Key Processes
While highly effective, using an inert atmosphere involves specific equipment and operational considerations. It is specified for processes where the benefits of a controlled environment are non-negotiable.
The Benefit: Superior Product Quality
By eliminating unwanted reactions, the final product exhibits higher material integrity, fewer impurities, and more consistent, predictable mechanical properties. This is critical in demanding industries like aerospace and medical.
The Benefit: Increased Efficiency
Parts emerge from the furnace clean and often ready for the next manufacturing step. This reduction in post-treatment processing saves significant time, labor, and cost, leading to a higher overall pass rate for finished goods.
The Cost: Equipment and Gas Supply
Inert atmosphere furnaces are more complex and expensive than their air-fired counterparts. They require a reliable, continuous supply of high-purity gas, which represents an ongoing operational cost.
Common Processes Requiring an Inert Atmosphere
- Bright Annealing: Softening a metal to improve its ductility without causing any surface discoloration.
- Sintering: Fusing metal powders together by heating them below their melting point. The vast surface area of the powders makes them extremely vulnerable to oxidation.
- Brazing: Joining two components using a filler metal. Clean, oxide-free surfaces are essential for the filler metal to flow and create a strong bond.
- Neutral Hardening: Hardening a steel component while ensuring its surface chemistry remains completely unchanged.
Making the Right Choice for Your Application
Selecting the right furnace atmosphere depends entirely on the material requirements of the finished part.
- If your primary focus is surface finish and appearance: An inert atmosphere is non-negotiable to prevent oxidation and achieve a bright, clean surface.
- If your primary focus is structural integrity: For applications like sintering or aerospace brazing, an inert atmosphere is critical to ensure clean, strong bonds and prevent internal defects.
- If your primary focus is controlled surface hardening: An inert gas is a necessary carrier to precisely control the chemical reaction at the material's surface.
- If your process is tolerant of surface scale: For applications like rough forging or basic stress relief where a final cleaning step is already planned, a simpler air furnace may be sufficient.
Ultimately, specifying an inert atmosphere is a deliberate engineering choice to gain absolute control over a material's chemistry at its most vulnerable state.
Summary Table:
| Key Aspect | Details |
|---|---|
| Purpose | Prevent unwanted chemical reactions (e.g., oxidation, decarburization) at high temperatures. |
| Common Gases Used | Nitrogen (N₂), Argon (Ar) |
| Benefits | Clean 'bright' finish, improved material integrity, reduced post-processing, precise surface chemistry control. |
| Common Processes | Bright Annealing, Sintering, Brazing, Neutral Hardening |
| Industries | Aerospace, Medical, Manufacturing |
Upgrade Your Heat Treatment with Precision and Control
At KINTEK, we understand the critical role of inert atmosphere furnaces in achieving flawless material properties. Our advanced solutions, including Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, are engineered to deliver unmatched performance and reliability. With our strong in-house R&D and manufacturing capabilities, we offer deep customization to meet your unique experimental and production needs—ensuring your processes are efficient, cost-effective, and free from contamination risks.
Don't let oxidation compromise your results. Contact us today to discuss how our high-temperature furnace solutions can elevate your lab's capabilities and drive innovation in your projects!
Visual Guide
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- What is nitrogen used for in a furnace? Prevent Oxidation and Control Heat Treatment Quality
- What does inert mean in furnace atmospheres? Protect materials from oxidation with inert gases.
- What is the main purpose of heat treatment? Transform Metal Properties for Superior Performance
- What are the environmental benefits of using inert gases in furnaces? Reduce Waste and Emissions for a Greener Process
- What is the use of nitrogen in furnace? Prevent Oxidation for Superior Heat Treatment



















