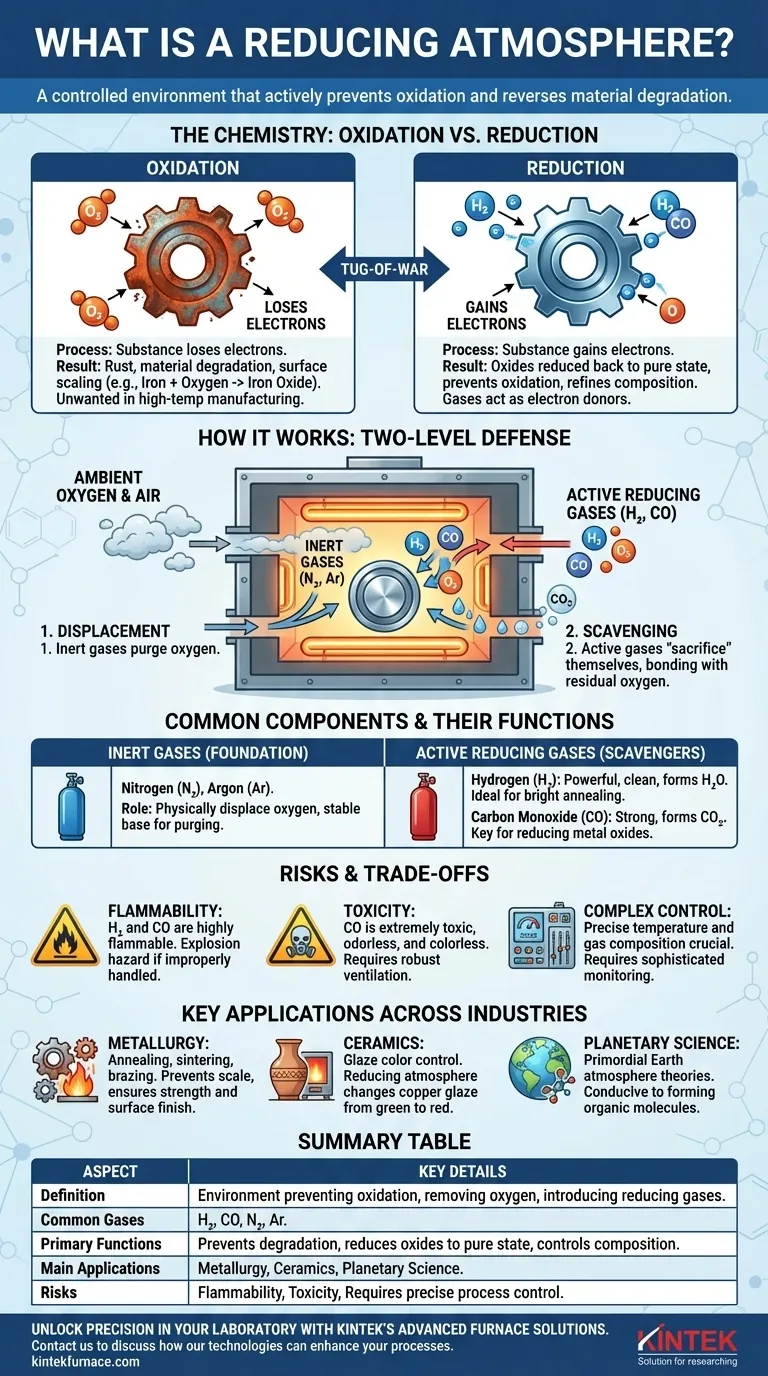
In short, a reducing atmosphere is a controlled environment that actively prevents oxidation—the chemical process that causes rust and other forms of material degradation. It achieves this by removing reactive oxygen and introducing specific gases, such as hydrogen or carbon monoxide, that readily bond with and neutralize any stray oxygen atoms, thereby protecting the target material.
The core purpose of a reducing atmosphere is not just to create an oxygen-free space, but to establish a chemically active environment that will reverse or "reduce" oxides back to their pure state. This shifts the goal from merely preventing damage to actively refining and controlling a material's chemical composition.

The Chemistry: Oxidation vs. Reduction
To grasp why a reducing atmosphere is so critical in many industrial and scientific processes, you must first understand the fundamental tug-of-war between oxidation and reduction.
The Nature of Oxidation
Oxidation is a chemical reaction where a substance loses electrons. While many elements can cause this, oxygen is the most famous oxidizing agent.
When iron rusts, it becomes iron oxide. The iron atoms have lost electrons to oxygen atoms, changing the material's properties from strong and metallic to brittle and flaky. This surface scaling is often undesirable in high-temperature manufacturing.
The Role of Reduction
Reduction is the exact opposite process: a substance gains electrons. An atmosphere that causes this is called a reducing atmosphere.
It contains gases known as reducing agents (e.g., hydrogen, carbon monoxide). These gases are electron donors; they readily give up their own electrons to "reduce" an oxidized material back to its elemental form or to prevent it from oxidizing in the first place.
How It Works in Practice
A reducing atmosphere functions as a two-level defense. First, it displaces ambient oxygen with an inert gas. Second, it adds an active reducing gas that acts as a scavenger.
This reducing gas is more reactive with oxygen than the material being protected. It essentially "sacrifices" itself by bonding with any residual oxygen, forming harmless byproducts like water (H₂O) or carbon dioxide (CO₂), leaving the target material untouched and clean.
Common Components and Their Functions
Creating a reducing atmosphere requires a precise mixture of gases, each with a specific role. The composition is tailored to the material being treated and the desired outcome.
Inert Gases: The Foundation
Gases like nitrogen (N₂) and argon (Ar) are often used as the base. They are chemically stable and serve to purge the furnace or chamber, physically displacing the oxygen-rich air. This is the first and most basic step.
Active Reducing Gases: The Scavengers
These are the active ingredients that define the atmosphere's reducing potential.
-
Hydrogen (H₂): A very powerful and clean reducing agent. It reacts with oxygen to form water vapor (H₂O), which can be easily vented. It is highly effective for processes like "bright annealing," which leaves metals with a mirror-like finish.
-
Carbon Monoxide (CO): Also a strong reducing agent. It reacts with oxygen to form carbon dioxide (CO₂). It is particularly effective at reducing certain metal oxides (like iron ore) and is a key component in many metallurgical processes.
Understanding the Trade-offs and Risks
While powerful, creating and maintaining a reducing atmosphere involves significant challenges and hazards that require strict engineering controls.
Flammability and Explosion Hazard
Hydrogen and carbon monoxide are both highly flammable. Improper handling, leaks, or incorrect gas-to-air ratios can lead to catastrophic explosions, especially in the high-temperature environments where these atmospheres are used.
Toxicity Concerns
Carbon monoxide is extremely toxic to humans, even at low concentrations. It is colorless and odorless, necessitating robust ventilation systems and continuous monitoring to ensure worker safety.
Process Control Complexity
Maintaining the precise temperature and gas composition is not trivial. Minor fluctuations can alter the chemical reactions, potentially damaging the product or even creating new, unwanted byproducts. This requires sophisticated sensors, feedback loops, and process control systems.
Key Applications Across Industries
Reducing atmospheres are not a niche concept; they are fundamental to many modern manufacturing and scientific fields.
Metallurgy and Metalworking
This is the most common application. In processes like annealing, sintering, and brazing, a reducing atmosphere prevents the formation of surface oxides (scale), ensuring the metal retains its desired strength, ductility, and surface finish.
Ceramics and Glazing
In pottery and ceramics, the kiln atmosphere dictates the final color of glazes. A copper-based glaze will turn green in an oxygen-rich (oxidizing) fire but a deep red in a reducing fire, as the copper oxide is "reduced" back to pure copper metal.
Early Earth and Planetary Science
Scientists believe Earth's primordial atmosphere was a reducing one, rich in methane, ammonia, and water vapor, with very little free oxygen. This concept is central to theories about the origin of life (abiogenesis), as such conditions are conducive to forming complex organic molecules.
Making the Right Choice for Your Goal
The decision to use a reducing atmosphere—and which kind—depends entirely on the desired outcome for your material.
- If your primary focus is preventing surface oxidation (scaling) on sensitive metals: A hydrogen-based reducing atmosphere is ideal for achieving a clean, "bright" finish.
- If your primary focus is bulk chemical transformation, like smelting ore: A carbon monoxide-rich atmosphere is a cost-effective and powerful choice for reducing metal oxides on a large scale.
- If your primary focus is simply displacing oxygen for a non-critical process: A simple inert atmosphere of nitrogen or argon may be sufficient, safer, and more economical.
By mastering the principles of atmospheric control, you gain the power to dictate the final chemical state and physical properties of your materials.
Summary Table:
| Aspect | Key Details |
|---|---|
| Definition | Environment that prevents oxidation by removing oxygen and introducing reducing gases. |
| Common Gases | Hydrogen (H₂), Carbon Monoxide (CO), Nitrogen (N₂), Argon (Ar). |
| Primary Functions | Prevents material degradation, reduces oxides to pure state, controls chemical composition. |
| Main Applications | Metallurgy (annealing, sintering), ceramics (glaze coloring), planetary science. |
| Risks | Flammability (H₂, CO), toxicity (CO), requires precise process control. |
Unlock Precision in Your Laboratory with KINTEK's Advanced Furnace Solutions
Leveraging exceptional R&D and in-house manufacturing, KINTEK provides diverse laboratories with advanced high-temperature furnace solutions. Our product line, including Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, is complemented by our strong deep customization capability to precisely meet unique experimental requirements. Whether you're in metallurgy, ceramics, or materials science, we deliver reliable, safe, and efficient systems tailored to your needs.
Contact us today to discuss how our reducing atmosphere technologies can enhance your processes and outcomes. Get in touch now!
Visual Guide
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- How does nitrogen atmosphere heat treatment improve surface strengthening? Enhance Durability and Performance
- What does inert mean in furnace atmospheres? Protect materials from oxidation with inert gases.
- How does the inert atmosphere heat treating process work? Prevent Oxidation for Superior Material Quality
- What is the use of nitrogen in furnace? Prevent Oxidation for Superior Heat Treatment
- What is nitrogen used for in a furnace? Prevent Oxidation and Control Heat Treatment Quality



















