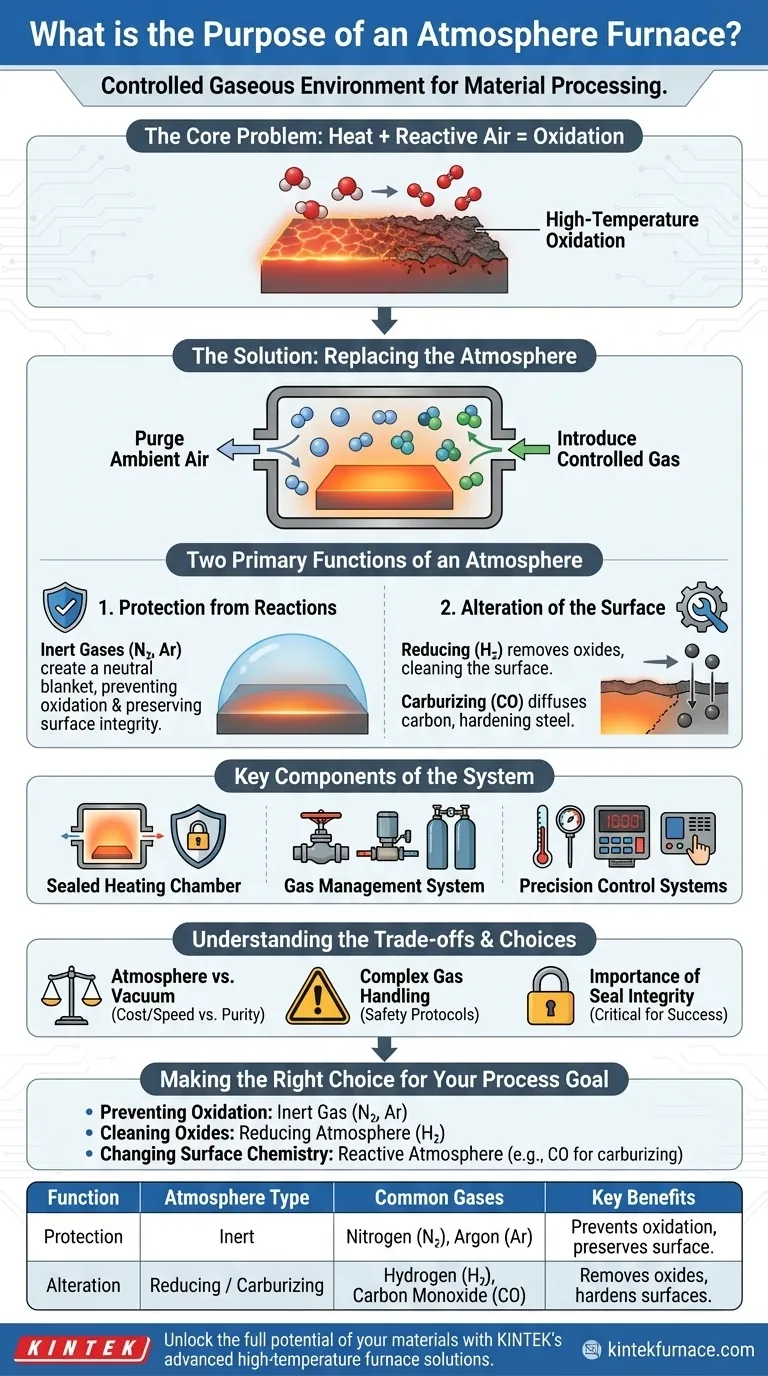
At its core, an atmosphere furnace is a high-temperature chamber that gives you complete control over the gaseous environment surrounding a material during processing. Instead of heating parts in ambient air, it allows you to introduce a specific, engineered atmosphere to achieve a desired outcome. The purpose is either to shield the material from unwanted chemical reactions like oxidation or to intentionally cause a specific chemical change on the material's surface.
The fundamental problem an atmosphere furnace solves is that high temperatures drastically accelerate chemical reactions. By replacing reactive air with a controlled gas, you gain precise command over the final properties and surface finish of your material, preventing damage or enabling specific surface engineering.

The Core Problem: Heat and Reactivity
Why Normal Air Is a Problem
At room temperature, the oxygen in the air reacts slowly with many materials, such as when iron rusts. When you heat that same material to hundreds or thousands of degrees, this process of oxidation accelerates dramatically.
This high-temperature oxidation can ruin a material's properties, create a brittle surface layer (scale), and compromise the integrity of the finished part.
The Solution: Replacing the Atmosphere
An atmosphere furnace solves this by first purging the ambient air from its sealed chamber. It then introduces a specific gas or gas mixture—the "atmosphere"—that will not harm the material.
This controlled environment ensures that the only changes happening to the material are the ones you intend from the heat treatment process itself, not from unwanted chemical side-reactions.
The Two Primary Functions of an Atmosphere
The purpose of the chosen atmosphere falls into one of two categories: protection or alteration.
Function 1: Protection from Reactions
This is the most common use. The goal is to create a neutral, non-reactive environment that shields the material from oxygen and other contaminants.
Inert gases like nitrogen (N₂) and argon (Ar) are ideal for this. They do not react with the material being heated, effectively creating a safe blanket that prevents oxidation and preserves the material's surface integrity.
Function 2: Alteration of the Surface
Sometimes, the goal isn't just to protect the surface, but to intentionally change its chemical composition. This is a powerful technique used in materials engineering.
A reducing atmosphere, which often contains hydrogen (H₂), can be used to actively remove oxides from a material's surface, effectively cleaning it at high temperatures.
Alternatively, a carburizing atmosphere, rich in carbon monoxide (CO), is used to diffuse carbon atoms into the surface of steel, significantly increasing its hardness.
Key Components of the System
The Sealed Heating Chamber
This is the core of the furnace, often configured as a box or a tube. It is built from materials that can withstand extreme heat and is designed with robust sealing mechanisms to prevent the controlled atmosphere from leaking out or air from leaking in.
The Gas Management System
This includes the gas inlets, outlets, and flow controllers. This system allows for the precise introduction, mixing, and exhausting of gases to create and maintain the specified atmospheric composition throughout the heating and cooling cycle.
Precision Control Systems
Modern furnaces rely on sophisticated controllers. Thermocouples measure the temperature with high accuracy, while the controller adjusts the heating elements. An atmosphere controller manages the gas flow rates and pressures, ensuring the environment remains stable and correct for the process.
Understanding the Trade-offs
Atmosphere Furnace vs. Vacuum Furnace
An atmosphere furnace is generally less expensive and has faster cycle times than a vacuum furnace. However, a vacuum furnace can achieve a higher level of purity by removing nearly all molecules, which is critical for extremely sensitive materials.
The Complexity of Gas Handling
Using an engineered atmosphere introduces complexity. Some gases, like hydrogen, are highly flammable and require stringent safety protocols. Others can be toxic. Proper handling, storage, and ventilation are non-negotiable safety requirements.
The Importance of Seal Integrity
The entire purpose of the furnace is defeated by a poor seal. Any leak allowing oxygen to enter the chamber during a high-temperature process can lead to catastrophic failure of the part. Constant monitoring and maintenance of seals are critical for reliable operation.
Making the Right Choice for Your Process
The atmosphere you choose is dictated entirely by your process goal.
- If your primary focus is simply preventing oxidation on a stable material: An inert gas like nitrogen or argon is your most straightforward and cost-effective choice.
- If your primary focus is cleaning existing surface oxides from a part: You will need a reducing atmosphere containing hydrogen to chemically reverse the oxidation.
- If your primary focus is changing the surface chemistry (e.g., hardening steel): A reactive atmosphere with specific constituents, like carbon monoxide for carburizing, is required.
Mastering atmosphere control transforms a furnace from a simple oven into a precise tool for materials engineering.
Summary Table:
| Function | Atmosphere Type | Common Gases | Key Benefits |
|---|---|---|---|
| Protection from Reactions | Inert | Nitrogen (N₂), Argon (Ar) | Prevents oxidation, preserves surface integrity |
| Alteration of the Surface | Reducing / Carburizing | Hydrogen (H₂), Carbon Monoxide (CO) | Removes oxides, hardens surfaces via carbon diffusion |
Unlock the full potential of your materials with KINTEK's advanced high-temperature furnace solutions. Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with tailored options like Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures precise alignment with your unique experimental needs. Contact us today to discuss how our furnaces can enhance your process efficiency and results!
Visual Guide
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- How does a chemically inert atmosphere function in a furnace? Prevent Oxidation and Ensure Material Purity
- How does the inert atmosphere heat treating process work? Prevent Oxidation for Superior Material Quality
- What are the benefits of inert atmosphere heat treating? Prevent Oxidation and Preserve Material Integrity
- What is nitrogen used for in a furnace? Prevent Oxidation and Control Heat Treatment Quality
- What is the main purpose of heat treatment? Transform Metal Properties for Superior Performance



















