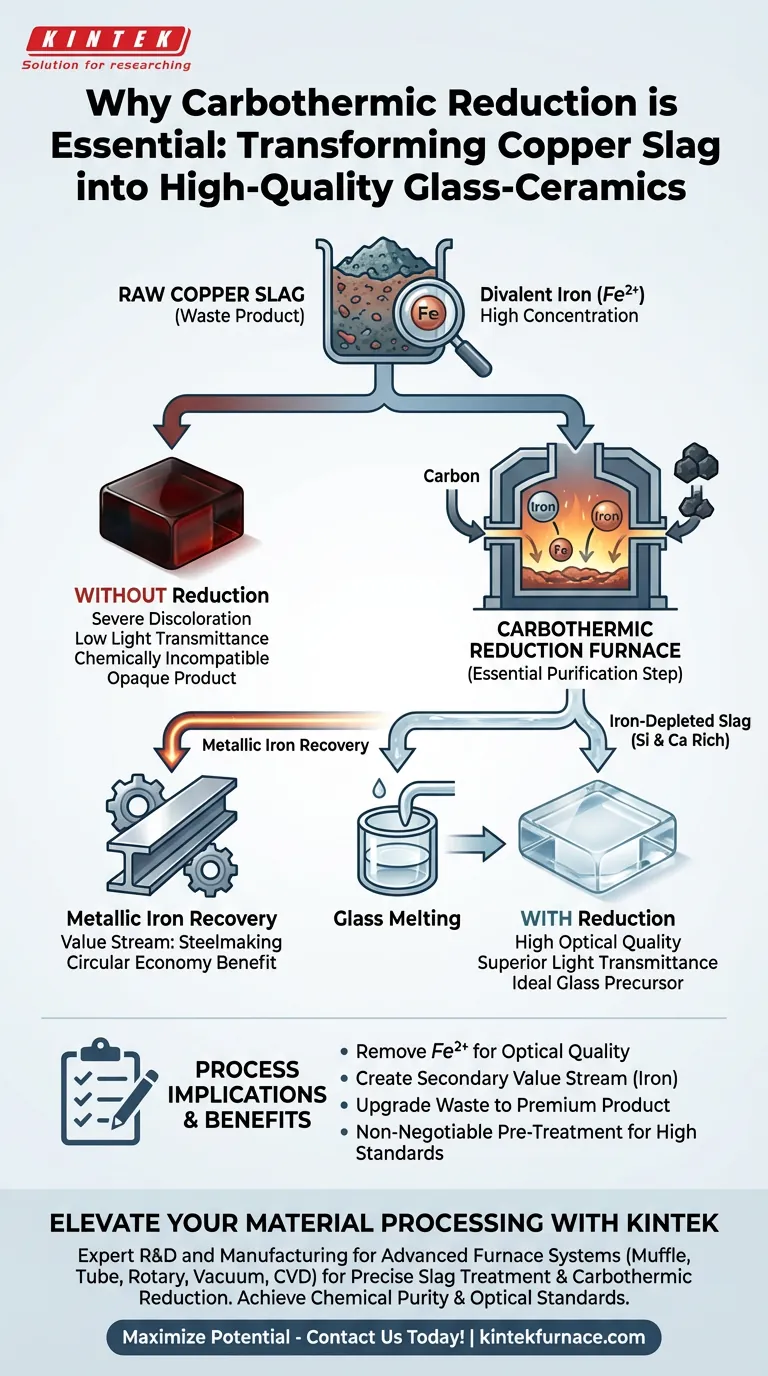
Carbothermic reduction is the essential purification step required to transform copper slag from a waste product into a viable raw material for glass-ceramics. By removing excessive amounts of divalent iron ($Fe^{2+}$) prior to the melting phase, this process prevents severe discoloration and ensures the final material achieves the necessary light transmittance and optical quality.
Copper slag is naturally abundant in iron, which degrades the optical properties of glass. Carbothermic reduction solves this by extracting the iron for use in steelmaking, leaving behind a purified, silicon-and-calcium-rich residue that is chemically ideal for manufacturing high-quality glass-ceramics.
The Chemistry of the Problem: Iron Contamination
The Role of Divalent Iron
Copper slag naturally contains high concentrations of divalent iron ($Fe^{2+}$). While common in slag, this specific chemical component is detrimental to glass manufacturing.
Impact on Optical Quality
The presence of $Fe^{2+}$ causes severe coloring issues within the glass matrix. This results in a drastic reduction of light transmittance, rendering the final glass-ceramic product opaque or heavily tinted rather than clear or controlled.
The Barrier to High Quality
Without removing this iron, the slag cannot be used for high-value applications. The material would fail to meet the aesthetic and functional standards required for commercial glass-ceramics.
The Carbothermic Solution
Separation via Reduction
To solve the iron problem, the slag undergoes treatment in a reduction furnace before the final glass melting process. This step utilizes carbon to chemically reduce the iron oxides, separating the metallic iron from the rest of the slag.
Creating a Secondary Value Stream
This process does not just remove a contaminant; it recovers a resource. The separated iron is harvested and directed toward steelmaking, adding economic value to the process.
The Ideal Precursor
Once the iron is removed, the remaining material is known as iron-depleted slag. This purified substance is rich in silicon and calcium, which are the primary structural components needed to produce high-quality glass-ceramics.
Understanding the Process Implications
The Necessity of Pre-Treatment
It is a common misconception that industrial slag can be used "as-is" for advanced materials. You must accept that raw copper slag is chemically incompatible with high-quality glass production without this intermediate reduction step.
Quality vs. Complexity
Implementing a reduction furnace adds a step to the manufacturing line. However, this is the non-negotiable trade-off required to upgrade a waste material into a premium industrial product.
Making the Right Choice for Your Goal
To maximize the value of copper slag in your production line, consider your specific objectives:
- If your primary focus is Optical Quality: You must prioritize the carbothermic reduction step to minimize divalent iron ($Fe^{2+}$) and ensure high light transmittance.
- If your primary focus is Circular Economy: Leverage the reduction process to separate iron for steelmaking, effectively creating two distinct product streams from one waste source.
By isolating the iron first, you unlock the slag's full potential as a silicon-rich foundation for advanced materials.
Summary Table:
| Process Component | Role/Impact in Glass-Ceramic Production |
|---|---|
| Raw Copper Slag | High in $Fe^{2+}$, causes opacity and severe discoloration |
| Carbothermic Reduction | Essential purification step using carbon to separate iron |
| Iron Recovery | Separated metallic iron is redirected to steelmaking value streams |
| Iron-Depleted Slag | Purified, silicon-calcium rich residue ideal for glass-ceramics |
| Final Product | Achieves high light transmittance and superior optical quality |
Elevate Your Material Processing with KINTEK
Ready to transform industrial by-products into high-value glass-ceramics? Backed by expert R&D and manufacturing, KINTEK offers advanced Muffle, Tube, Rotary, Vacuum, and CVD systems, including specialized high-temperature furnaces perfect for carbothermic reduction and precise slag treatment. Our customizable lab solutions are designed to help you achieve the exact chemical purity and optical standards your projects demand.
Maximize your lab's efficiency and material potential—contact us today to discuss your unique processing needs!
Visual Guide
References
- Jiaxing Liu, Baisui Han. The Utilization of the Copper Smelting Slag: A Critical Review. DOI: 10.3390/min15090926
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Ultra High Vacuum CF Flange Stainless Steel Sapphire Glass Observation Sight Window
- Ultra High Vacuum Observation Window KF Flange 304 Stainless Steel High Borosilicate Glass Sight Glass
- Ultra High Vacuum CF Observation Window Flange with High Borosilicate Glass Sight Glass
- Chairside Dental Porcelain Zirconia Sintering Furnace with Transformer for Ceramic Restorations
People Also Ask
- How does a laboratory air furnace contribute to the pre-oxidation stage of electrospun nanofibers? Expert Guide
- What is the composition of a typical endothermic atmosphere used for heat treating steel? Optimize Your Steel Heat Treatment Process
- What are the common applications of program-controlled atmosphere furnaces? Essential for High-Temp Material Processing
- What are the cost considerations when using argon in heat treatment? Maximize Savings and Quality
- What is an atmosphere box furnace and what are its primary uses? Essential for Controlled Heat Processing
- What are the key benefits of precise temperature control in a controlled atmosphere furnace? Unlock Superior Quality and Efficiency
- For which materials is the experimental box type atmosphere furnace suitable? Ideal for Metals, Ceramics, and Advanced Materials
- What is the primary purpose of an inert oven? Protect Materials from Oxidation in Heating



