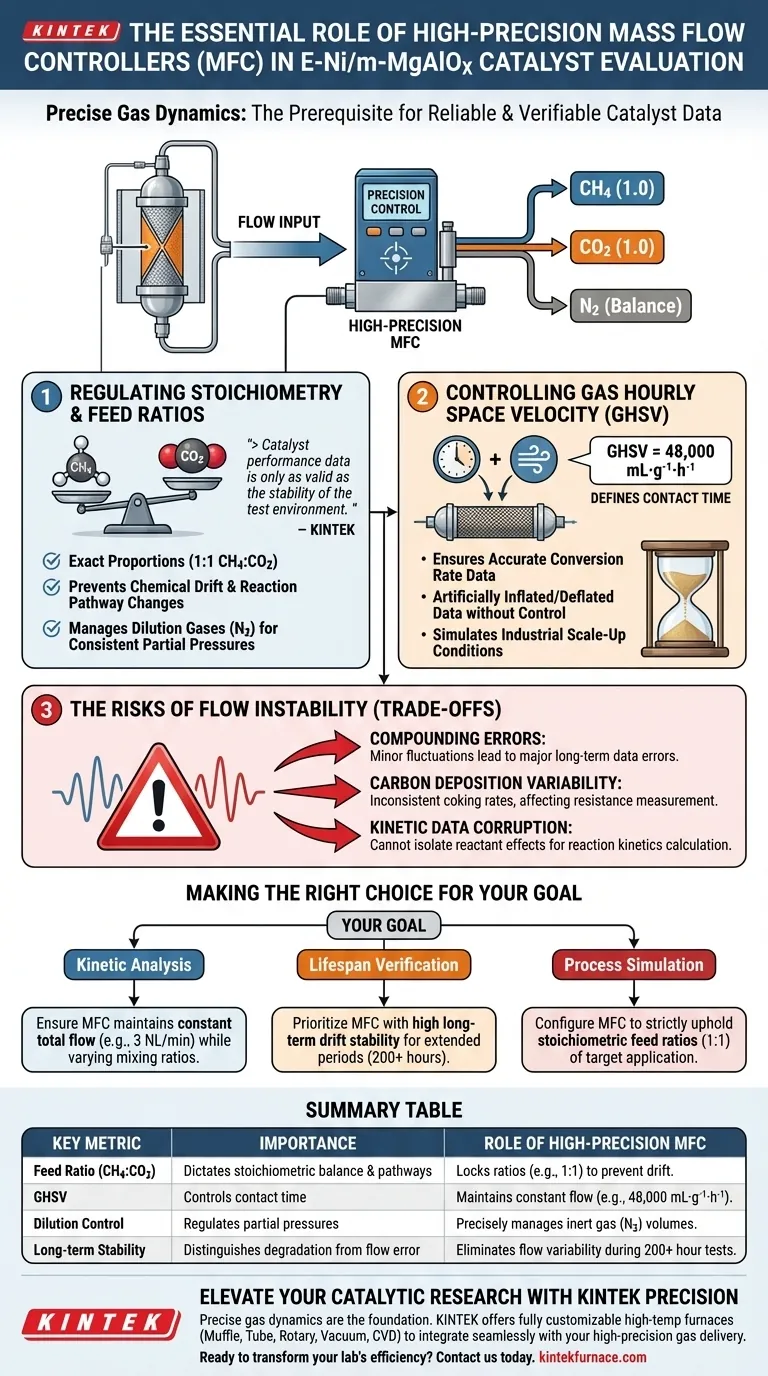
Precise control of gas dynamics is the prerequisite for reliable catalyst evaluation. To evaluate E-Ni/m-MgAlOx catalysts effectively, you must maintain exact feed ratios of methane, carbon dioxide, and nitrogen while strictly regulating Gas Hourly Space Velocities (GHSV). A high-precision Mass Flow Controller (MFC) is the only instrument capable of maintaining these specific proportions—such as a 1:1 CH4 to CO2 ratio—to ensure experimental repeatability.
Catalyst performance data is only as valid as the stability of the test environment. A high-precision MFC eliminates flow rate variability, ensuring that observed changes in conversion rates are strictly due to the catalyst's behavior, not inconsistent gas delivery.

Regulating Stoichiometry and Feed Ratios
The Necessity of Exact Proportions
Catalytic reforming reactions depend heavily on specific chemical balances. For E-Ni/m-MgAlOx catalysts, maintaining a precise ratio, often 1:1 of Methane (CH4) to Carbon Dioxide (CO2), is critical.
Preventing Chemical Drift
If the feed ratio fluctuates, the fundamental chemistry of the reaction changes. An MFC locks these ratios in, preventing deviations that would alter the reaction pathway or product distribution.
Managing Dilution Gases
Nitrogen (N2) is often used as a balance gas. An MFC regulates the exact volume of this inert gas to maintain consistent partial pressures of the active reactants.
Controlling Gas Hourly Space Velocity (GHSV)
Defining Contact Time
GHSV determines how much gas contacts a specific weight of catalyst over time (e.g., 48,000 mL·g⁻¹·h⁻¹). This metric dictates the "contact time" between the reactants and the catalytic surface.
Ensuring Conversion Accuracy
If the flow rate drifts, the contact time changes. This artificially inflates or deflates conversion rate data, making it impossible to accurately judge the catalyst's efficiency.
Impact on Scale-Up
precise GHSV control allows researchers to simulate industrial conditions. This data is vital for predicting how the catalyst will perform when scaled up from a lab reactor to a commercial facility.
The Risks of Flow Instability (Trade-offs)
Compounding Errors Over Time
In long-term stability testing—which can last hundreds of hours or even days—minor flow fluctuations compound into major data errors. Without an MFC, you cannot distinguish between actual catalyst degradation and simple inconsistencies in gas supply.
Carbon Deposition Variability
Inconsistent flow rates can alter carbon deposition (coking) rates. To accurately measure how resistant a catalyst is to coking, the flow environment must remain perfectly static.
Kinetic Data Corruption
To calculate reaction kinetics, you must isolate specific variables. If the total flow rate fluctuates, you cannot isolate the effect of methane partial pressure on hydrogen production.
Making the Right Choice for Your Goal
To ensure your data withstands scrutiny, align your flow control strategy with your specific experimental objectives:
- If your primary focus is Kinetic Analysis: Ensure your MFC can maintain a constant total flow (e.g., 3 NL/min) while precisely varying the mixing ratios of methane and dilution gases.
- If your primary focus is Lifespan Verification: Prioritize an MFC with high long-term drift stability to maintain a specific GHSV over extended testing periods (e.g., 200+ hours).
- If your primary focus is Process Simulation: configure the MFC to strictly uphold the stoichiometric feed ratios (1:1) found in target industrial applications.
Precision in flow control turns qualitative observations into quantitative, verifiable science.
Summary Table:
| Key Metric | Importance in Catalyst Evaluation | Role of High-Precision MFC |
|---|---|---|
| Feed Ratio (CH4:CO2) | Dictates stoichiometric balance & reaction pathways | Locks ratios (e.g., 1:1) to prevent chemical drift |
| GHSV | Controls contact time between gas and catalyst | Maintains constant flow (e.g., 48,000 mL·g⁻¹·h⁻¹) for accurate conversion data |
| Dilution Control | Regulates partial pressures of active reactants | Precisely manages inert gas (N2) volumes for balance |
| Long-term Stability | Distinguishes catalyst degradation from flow error | Eliminates flow rate variability during 200+ hour lifespan tests |
Elevate Your Catalytic Research with KINTEK Precision
Precise gas dynamics are the foundation of verifiable catalyst evaluation. At KINTEK, we understand that even minor flow fluctuations can corrupt your kinetic data and stability results.
Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of lab high-temp furnaces—including Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to integrate seamlessly with your high-precision gas delivery needs. Whether you are performing kinetic analysis or industrial process simulation, our systems provide the stable thermal environment required to match your MFC's precision.
Ready to transform your lab's efficiency? Contact us today to discuss your unique catalyst testing requirements with our technical team.
Visual Guide
References
- Kyung Hee Oh, Ji Chan Park. Scalable Exsolution‐Derived E‐Ni/m‐MgAlO <sub>x</sub> Catalysts with Anti‐Sintering Stability for Methane Dry Reforming. DOI: 10.1002/smll.202508028
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 304 316 Stainless Steel High Vacuum Ball Stop Valve for Vacuum Systems
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Ultra High Vacuum Stainless Steel KF ISO CF Flange Pipe Straight Pipe Tee Cross Fitting
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- Why is vacuum sealing in quartz tubes essential for Cr0.82Mn0.18Ge? Ensure Stoichiometry & Purity
- How does a laboratory blast drying oven facilitate the treatment of Au/ZnO/In2O3 precursor precipitates? Key Benefits
- Why is high-purity tantalum foil used when melting Ce2(Fe, Co)17 alloy? Protect Your Rare-Earth Materials
- What is the purpose of using quartz vacuum encapsulation? Optimize La(Fe,Si)13-based Magnetocaloric Alloys
- What are the alternative names for a Laboratory Furnace? Find the Right High-Temperature Tool for Your Lab
- How many taps does the water circulating vacuum pump have? Choose the Right Model for Your Lab
- Why are high-purity alumina grinding balls used for Al2O3/TiC milling? Master Chemical Consistency
- What factors affect the light transmittance of alumina tubes? Balance Clarity and Durability for Your Lab



















