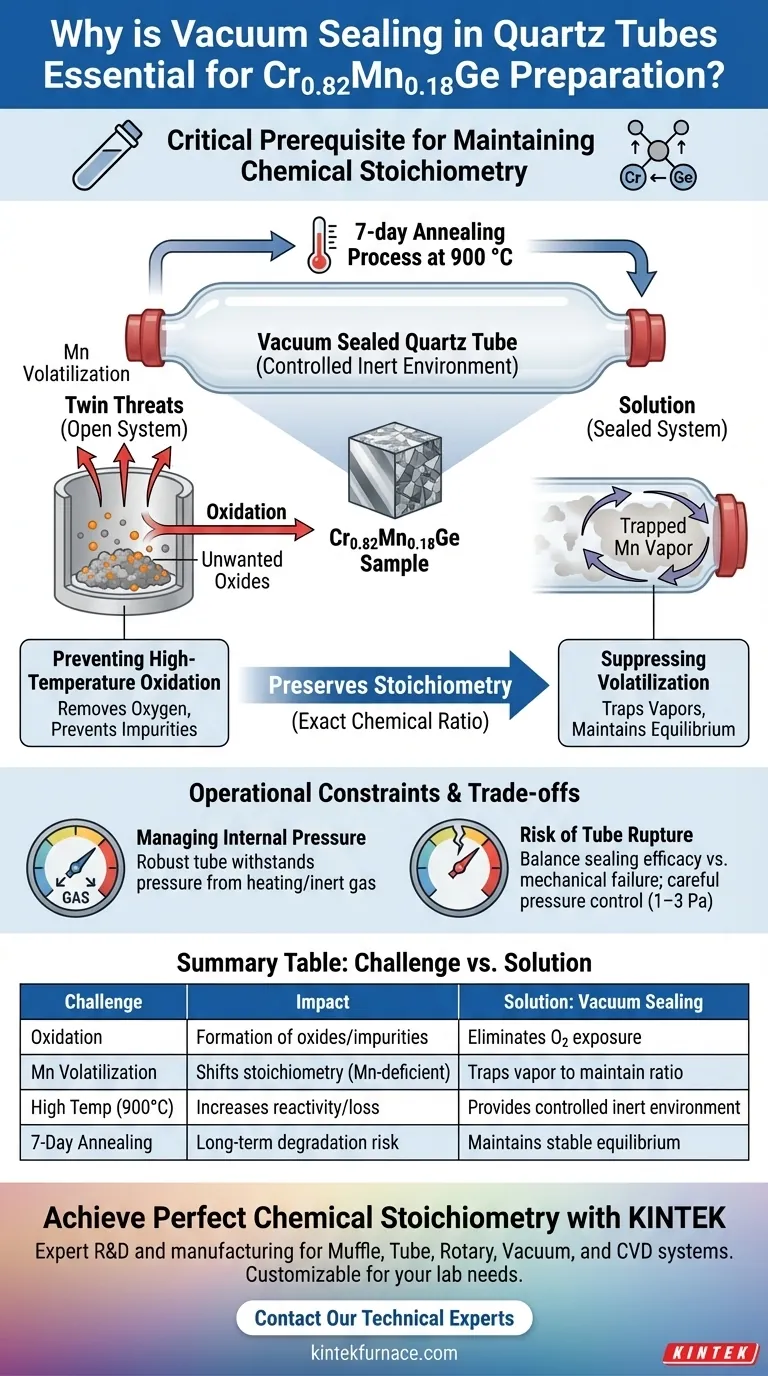
Vacuum sealing in quartz tubes is a critical prerequisite for synthesizing Cr0.82Mn0.18Ge to ensure the material retains its exact chemical composition. This step establishes a controlled, inert environment—often backfilled with partial argon—that protects the sample during the rigorous 7-day annealing process at 900 °C.
The primary function of this encapsulation is to strictly maintain chemical stoichiometry. By creating a closed system, the process eliminates oxygen exposure and suppresses the evaporation of volatile elements, ensuring the final polycrystalline sample matches the intended formula.

The Twin Threats to Synthesis Stability
To understand why this step is non-negotiable, you must look at what happens to these specific elements when exposed to high temperatures in an open environment.
Preventing High-Temperature Oxidation
At 900 °C, metallic elements become highly reactive. Without a protective barrier, the components of the sample would react immediately with atmospheric oxygen.
Vacuum sealing removes oxygen from the equation. By evacuating the air and often replacing it with an inert gas like argon, the quartz tube prevents the formation of unwanted oxides that would contaminate the sample and disrupt the crystal structure.
Suppressing Volatilization of Manganese
The most critical challenge in this specific synthesis is the behavior of manganese (Mn).
Manganese is an "active" metallic element with a tendency to volatilize (turn into vapor) at high temperatures. In an open vessel, Mn atoms would escape into the furnace atmosphere.
The sealed quartz tube traps these vapors within a confined space. This saturation creates a state of equilibrium that prevents further loss of material from the solid sample.
Preserving Stoichiometry
The formula Cr0.82Mn0.18Ge relies on a precise ratio of atoms.
If the sample oxidizes, you introduce an impurity. If the manganese volatilizes, the ratio shifts, and the material becomes Mn-deficient. The vacuum seal ensures that the input mass matches the output mass, preserving the chemical stoichiometry required for the material's specific magnetic or electronic properties.
Operational Constraints and Trade-offs
While vacuum sealing is effective, it introduces specific physical constraints that must be managed to ensure safety and success.
Managing Internal Pressure
The "vacuum" is rarely a true void; it often contains partial pressure of argon. As the tube heats to 900 °C, the gas inside expands.
The quartz tube acts as a pressure vessel. It must be robust enough to withstand the internal pressure generated by the heating of the inert gas and any volatile components, yet thin enough to allow heat transfer.
The Risk of Tube Rupture
There is a tangible trade-off between sealing efficacy and mechanical failure.
If the internal pressure becomes too high—or if the quartz has micro-fractures—the tube can rupture. This not only ruins the sample by exposing it to air but can also damage the furnace. Proper preparation requires careful control of the initial argon fill pressure (typically 1–3 Pa in similar contexts) to account for thermal expansion.
Ensuring Synthesis Success
To apply this to your preparation of Cr0.82Mn0.18Ge, evaluate your process based on your specific stability goals.
- If your primary focus is phase purity: Ensure the vacuum level is high before backfilling with argon to eliminate all traces of reactive oxygen.
- If your primary focus is stoichiometric accuracy: Prioritize the integrity of the seal to prevent even microscopic leaks of manganese vapor over the 7-day annealing period.
A perfectly sealed quartz environment is not just a container; it is the active control mechanism that makes the formation of this precise compound possible.
Summary Table:
| Challenge | Impact on Synthesis | Solution: Vacuum Sealing |
|---|---|---|
| Oxidation | Formation of unwanted oxides and impurities | Eliminates oxygen/air exposure |
| Mn Volatilization | Shifts stoichiometry; Mn-deficient sample | Traps vapor to maintain chemical ratio |
| High Temp (900°C) | Increases elemental reactivity/loss | Provides a controlled, inert environment |
| 7-Day Annealing | Long-term degradation risk | Maintains stable equilibrium over time |
Achieve Perfect Chemical Stoichiometry with KINTEK
Precise material synthesis like Cr0.82Mn0.18Ge requires more than just a furnace—it requires a controlled environment that prevents oxidation and volatile element loss. KINTEK specializes in providing the specialized high-temperature equipment needed for rigorous research applications.
Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet your unique laboratory needs. Whether you are performing long-term annealing or complex vacuum sealing, our systems ensure the stability and purity your results demand.
Ready to elevate your synthesis process? Contact our technical experts today to find the perfect thermal solution for your lab.
Visual Guide
References
- Victor Ukleev, L. Caron. Observation of magnetic skyrmion lattice in Cr0.82Mn0.18Ge by small-angle neutron scattering. DOI: 10.1038/s41598-025-86652-1
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Magnesium Extraction and Purification Condensing Tube Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- What are the primary functions of high-purity graphite molds in SPS? Optimize Your Spark Plasma Sintering Process
- What role does a high-purity Graphite Crucible play in super-gravity zinc recovery? Key Benefits & Functions
- How is the vacuuming operation performed with a water circulating vacuum pump? Master the Liquid Ring Technique
- What is the point of a vacuum chamber? Achieve Absolute Control in Your Processes
- What roles do high-purity graphite dies play in SPS of Ti-6Al-4V? Mastering Efficient Composite Sintering
- What is the primary purpose of a benchtop blast drying oven? Optimize Barium Titanate Ceramic Preparation
- Why are alumina boats used for Bi2Se3 deposition? Ensure High-Purity Synthesis for Topological Insulators
- What is the function of a rotary vane vacuum pump in a thermal vacuum mercury removal system? Enhance Evaporative Efficiency

















