High flow rates are essential to prevent oxygen starvation at the reaction site, which would otherwise corrupt experimental data. By maintaining a robust flow, such as 400 mL/min, you ensure that the supply of oxygen never becomes the bottleneck, allowing the experiment to measure the true reaction properties of the magnetite rather than the limitations of the gas supply.
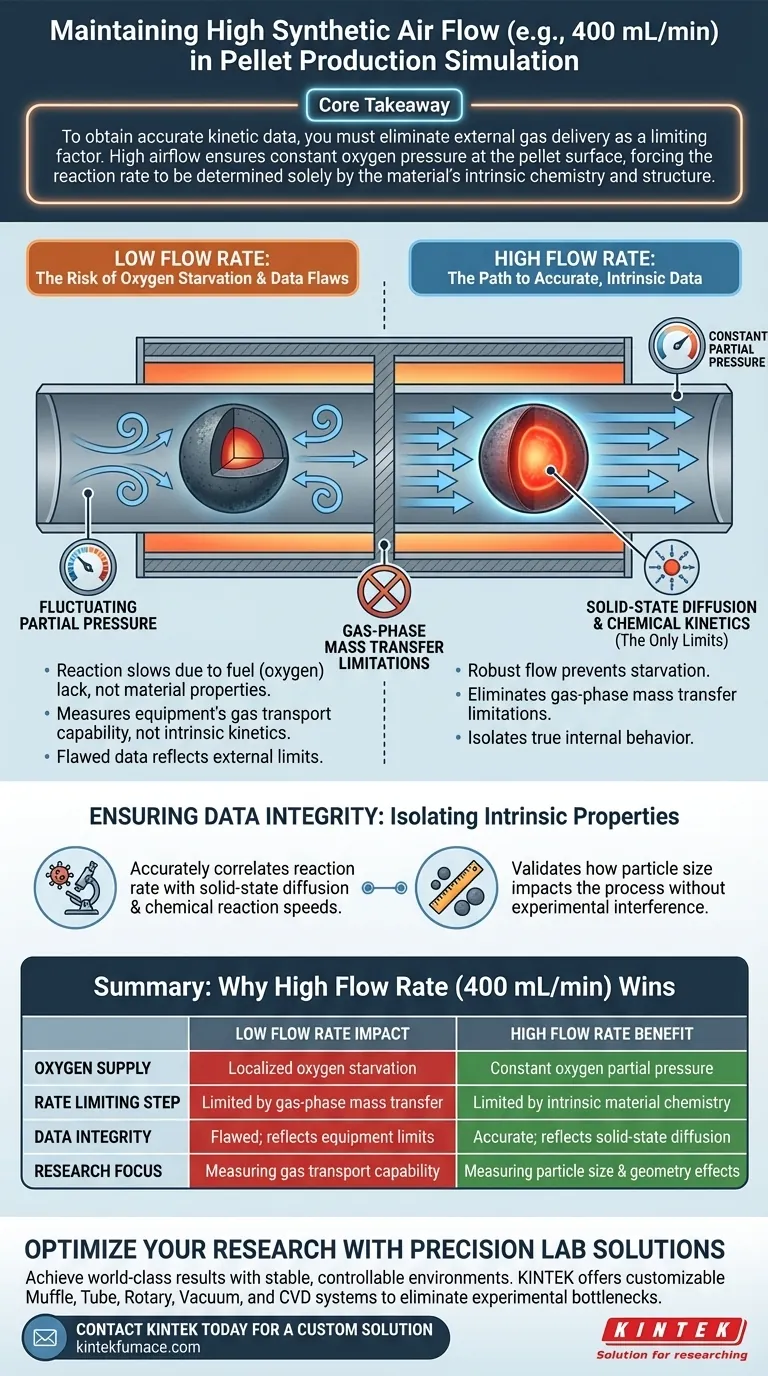
Core Takeaway To obtain accurate kinetic data, you must eliminate external gas delivery as a limiting factor. High airflow ensures constant oxygen pressure at the pellet surface, forcing the reaction rate to be determined solely by the material’s intrinsic chemistry and structure.

The Mechanics of Oxygen Supply
Preventing Oxygen Starvation
During the oxidation of magnetite to hematite, the reaction consumes oxygen rapidly. If the air supply is insufficient, the immediate area around the pellet suffers from oxygen starvation.
This creates a local environment where the reaction slows down not because of the material's properties, but simply because there is no fuel (oxygen) available to continue the process.
Stabilizing Partial Pressure
Accurate kinetic modeling requires stable variables. A high flow rate ensures that the oxygen partial pressure remains constant at the reaction interface.
Without this constant pressure, the driving force of the oxidation would fluctuate, introducing noise into your data that is difficult to isolate from the actual chemical kinetics.
Ensuring Data Integrity
Eliminating Mass Transfer Limitations
In kinetic experiments, there are generally two ways gas transport can limit the reaction rate: external (gas-phase mass transfer) and internal (solid-state diffusion).
A high flow rate effectively eliminates gas-phase mass transfer limitations. This ensures that the gas moves to the surface faster than the reaction can consume it.
Isolating Intrinsic Properties
Once external gas limitations are removed, the measured data reflects the true internal behavior of the pellet.
This allows you to accurately correlate the reaction rate with solid-state diffusion and chemical reaction speeds. It ensures that the influence of particle size on the oxidation process is captured accurately, without interference from the experimental setup.
Understanding the Trade-offs: External vs. Internal Control
The Risk of the Wrong Rate-Limiting Step
The critical trade-off in this experimental design is between measuring external airflow mechanics and internal material properties.
If the flow rate is too low, your data measures how fast the machine delivers air, not how the magnetite reacts. You are effectively measuring the gas transport capability of your equipment.
The Goal of Kinetic Analysis
By keeping the flow rate high (e.g., 400 mL/min), you shift the "rate-limiting step" to the material itself.
This creates a controlled environment where the only variables slowing down the reaction are the chemical kinetics and the physical structure of the pellet. This is the only way to validate how particle size impacts the process.
Making the Right Choice for Your Goal
To ensure your magnetite oxidation simulations yield valid industrial data, apply these principles:
- If your primary focus is accurate kinetic modeling: Maintain high flow rates to guarantee that the reaction is controlled by solid-state diffusion or chemical kinetics, not gas supply.
- If your primary focus is studying particle size effects: Ensure the oxygen partial pressure is constant at the interface so that changes in rate can be attributed strictly to particle geometry.
Ultimately, high airflow acts as an experimental control, rendering the external environment invisible so the true material behavior can be observed.
Summary Table:
| Factor | Low Flow Rate Impact | High Flow Rate (400 mL/min) Benefit |
|---|---|---|
| Oxygen Supply | Localized oxygen starvation | Constant oxygen partial pressure |
| Rate Limiting Step | Limited by gas-phase mass transfer | Limited by intrinsic material chemistry |
| Data Integrity | Flawed; reflects equipment limits | Accurate; reflects solid-state diffusion |
| Research Focus | Measuring gas transport capability | Measuring particle size & geometry effects |
Optimize Your Research with Precision Lab Solutions
To achieve world-class results in magnetite oxidation and pellet production simulations, your equipment must provide stable, controllable environments. Backed by expert R&D and manufacturing, KINTEK offers a wide range of high-performance lab equipment, including Muffle, Tube, Rotary, Vacuum, and CVD systems.
Our furnaces are fully customizable to your unique research needs, ensuring you eliminate experimental bottlenecks and isolate true material properties. Let our specialists help you select the perfect high-temperature system to maintain precise gas flow and thermal control.
Contact KINTEK Today for a Custom Solution
Visual Guide
References
- A. Laarich, Kurt N. Wiegel. Effect of Particle Size on Magnetite Oxidation Behavior: A Modeling Approach Incorporating Ultra-Fine Particle Effects. DOI: 10.1007/s11663-025-03640-6
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Magnesium Extraction and Purification Condensing Tube Furnace
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- What processes can continuous furnaces perform in a single step? Master Debinding and Sintering for High-Volume Production
- How does the control of gas flow and reaction time affect NiMo catalyst carbon layers? Master Nanostructure Engineering
- How does high-temperature calcination affect kaolin? Boost Surface Area and Catalytic Reactivity via Thermal Processing
- What is the primary role of the Thermal Oxidation (TO) process in Ti-6Al-4V ELI alloy? Enhancing Hardness and Wear
- What thermochemical environment does an entrained flow reactor provide? Simulate Industrial Biomass Combustion
- Why is a vacuum drying oven essential in the synthesis of CuCl nano-arrays? Protect Purity and Performance
- How does a bias power supply influence AlCrSiWN coatings? Master Ion Bombardment for Superior Durability
- What are the primary applications of vacuum chambers? Unlock Precision in Research and Manufacturing



















