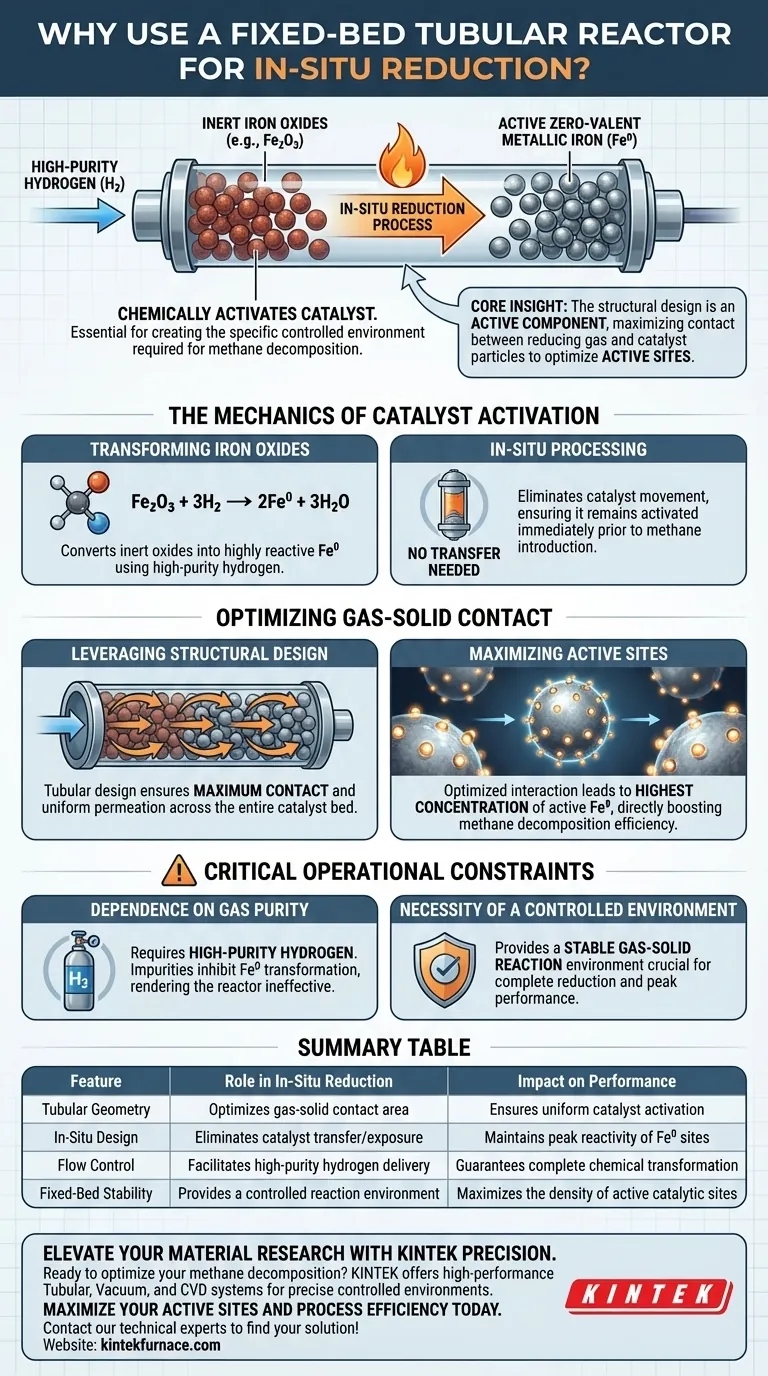
A fixed-bed tubular reactor is essential because it creates the specific controlled environment required to chemically activate the catalyst before the main reaction occurs. By passing high-purity hydrogen through the reactor, the system performs an in-situ reduction that transforms inert iron oxides into active zero-valent metallic iron (Fe0).
Core Insight: The structural design of the fixed-bed tubular reactor is not merely a vessel; it is an active component in maximizing the contact between the reducing gas and the catalyst particles. This specific configuration is required to optimize the concentration of active sites, ensuring the catalyst is fully primed for efficient methane decomposition.

The Mechanics of Catalyst Activation
Transforming Iron Oxides
The primary purpose of this phase is chemical transformation. The catalyst material typically exists as iron oxides, which are not yet reactive for methane decomposition.
To correct this, high-purity hydrogen is introduced into the reactor. This gas triggers a reduction reaction, converting the oxides into zero-valent metallic iron (Fe0).
The Role of In-Situ Processing
This reduction happens "in-situ," meaning inside the reactor where the final processing will take place.
This eliminates the need to move the catalyst between different vessels. It ensures the catalyst remains in its activated state immediately prior to the introduction of methane.
Optimizing Gas-Solid Contact
Leveraging Structural Design
The efficiency of the reduction process relies heavily on the physical geometry of the reactor.
The tubular design is engineered to ensure maximum contact between the hydrogen gas and the solid catalyst particles. This intimate contact is necessary to ensure the reduction reaction permeates the entire catalyst bed.
Maximizing Active Sites
The ultimate goal of this contact is to maximize the density of active sites on the catalyst surface.
By optimizing the gas-solid interaction, the reactor ensures that the highest possible concentration of iron is converted to its active metallic form. This directly correlates to the efficiency of the subsequent methane decomposition.
Critical Operational Constraints
Dependence on Gas Purity
The reference explicitly notes the use of high-purity hydrogen.
The effectiveness of the fixed-bed reactor is contingent on the quality of the reduction gas. Impurities in the hydrogen stream could inhibit the transformation to Fe0, rendering the structural advantages of the reactor moot.
The Necessity of a Controlled Environment
The fixed-bed reactor provides a "controlled gas-solid reaction environment."
This implies that without the stability provided by this specific reactor type, maintaining the conditions necessary for complete reduction would be difficult. Incomplete reduction leads to fewer active sites and lower overall system performance.
Ensuring Process Readiness
To derive the most value from a fixed-bed tubular reactor during the reduction phase, consider the following technical priorities:
- If your primary focus is Catalyst Activity: Ensure the reduction phase is sufficient to fully convert iron oxides to zero-valent metallic iron (Fe0) to maximize active sites.
- If your primary focus is Process Efficiency: Rely on the reactor's tubular design to facilitate maximum gas-solid contact, ensuring no portion of the catalyst bed is bypassed.
The success of methane decomposition is predetermined by how effectively the fixed-bed reactor facilitates this initial reduction and activation step.
Summary Table:
| Feature | Role in In-Situ Reduction | Impact on Performance |
|---|---|---|
| Tubular Geometry | Optimizes gas-solid contact area | Ensures uniform catalyst activation |
| In-Situ Design | Eliminates catalyst transfer/exposure | Maintains peak reactivity of Fe0 sites |
| Flow Control | Facilitates high-purity hydrogen delivery | Guarantees complete chemical transformation |
| Fixed-Bed Stability | Provides a controlled reaction environment | Maximizes the density of active catalytic sites |
Elevate Your Material Research with KINTEK Precision
Ready to optimize your methane decomposition and catalyst activation? Backed by expert R&D and manufacturing, KINTEK offers high-performance Tubular, Vacuum, and CVD systems designed to provide the precise controlled environments your processes demand. Whether you need a standard fixed-bed setup or a fully customized high-temperature furnace, our systems ensure maximum gas-solid contact and process stability for your lab.
Maximize your active sites and process efficiency today. Contact our technical experts to find your solution!
Visual Guide
References
- Hamid Ahmed, Ahmed S. Al‐Fatesh. Methane Decomposition over a Titanium-Alumina and Iron Catalyst Assisted by Lanthanides to Produce High-Performance COx-Free H2 and Carbon Nanotubes. DOI: 10.3390/catal15010077
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
People Also Ask
- What is the role of an inert gas protection tube furnace in hardening High Vanadium HSS? Achieve Precision Hardness
- What is the temperature of a quartz tube furnace? Key Limits and Application Insights
- What are the key features of a drop tube furnace? Unlock Precise High-Temperature Processing
- What factors should be considered when selecting a high temperature tube furnace? Ensure Precision and Reliability for Your Lab
- Why is the encapsulation of raw materials in a vacuum-sealed quartz tube necessary for crystal growth? Key to Purity
- How does a tube atmosphere furnace facilitate local CVD during PAN fiber carbonization? Master In-Situ CNT Growth
- What function does a tube furnace serve in the PVT growth of J-aggregate molecular crystals? Mastery of Thermal Control
- How do you clean an alumina tube furnace? Ensure Peak Performance & Longevity



















