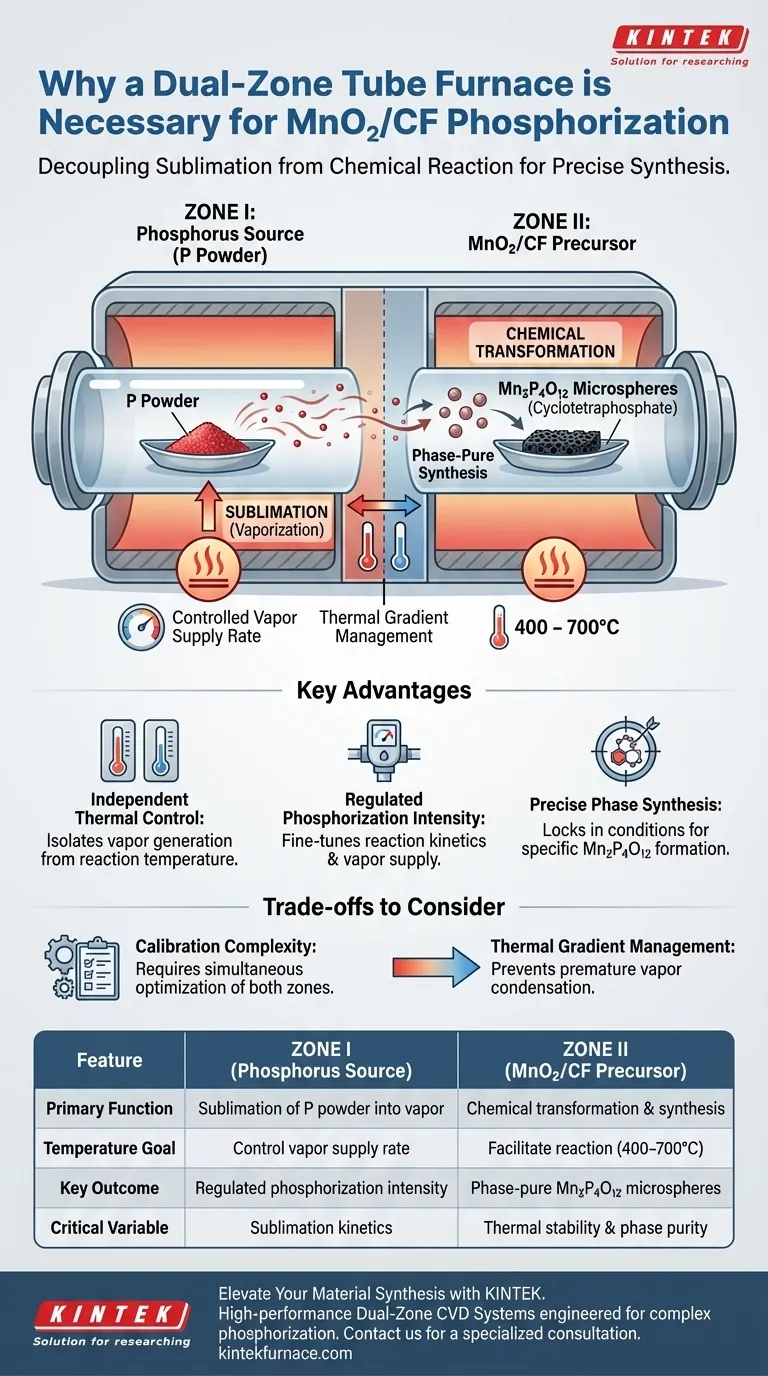
A dual-zone tube furnace provides the independent thermal control necessary to decouple the sublimation of the phosphorus source from the chemical reaction of the precursor. By physically separating the phosphorus powder (Zone I) from the MnO2/CF precursor (Zone II), the system allows for the precise regulation of phosphorization intensity, which is critical for converting the precursor into specific phases of cyclotetraphosphate (Mn2P4O12).
The necessity of a dual-zone system lies in its ability to isolate the generation of phosphorus vapor from the synthesis temperature of the target material. This separation allows for the fine-tuning of reaction kinetics, ensuring the successful synthesis of specific microsphere phases without thermal interference between the source and the substrate.

The Mechanics of Space-Confined CVD
Physical Separation of Components
In this Chemical Vapor Deposition (CVD) setup, the reactants are not mixed prior to heating.
Zone I is dedicated exclusively to the phosphorus source (P powder).
Zone II houses the target material, the MnO2/CF precursor.
This physical separation is the prerequisite for managing the distinct chemical behaviors of each material.
Independent Temperature Regimes
The core advantage of this configuration is the ability to maintain two different thermal environments simultaneously.
The phosphorus source requires a specific temperature to achieve the correct rate of sublimation (turning solid powder into vapor).
Conversely, the MnO2/CF precursor requires a different reaction temperature range, specifically 400 to 700°C, to facilitate the chemical transformation.
A dual-zone furnace ensures that the temperature required to vaporize the phosphorus does not dictate or compromise the temperature required to react the precursor.
Precision in Phase Synthesis
Regulating Phosphorization Intensity
The "phosphorization intensity" refers to how aggressively the phosphorus reacts with the MnO2/CF.
This intensity is driven by the concentration of phosphorus vapor reaching Zone II.
By adjusting the temperature of Zone I independently, you control the vapor supply rate without altering the reaction conditions in Zone II.
Achieving Specific Chemical Phases
The ultimate goal of this process is to synthesize cyclotetraphosphate (Mn2P4O12) microspheres.
The formation of this specific phase is highly sensitive to thermal conditions.
The dual-zone setup allows you to lock in the precise "recipe" of vapor density and reaction heat needed to stabilize this specific phase, rather than a random mixture of byproducts.
Understanding the Trade-offs
Calibration Complexity
While a dual-zone system offers superior control, it introduces more variables to the experimental process.
You must determine the optimal temperature for both zones simultaneously; an error in Zone I (vapor supply) can ruin the results in Zone II (reaction), even if Zone II is set correctly.
Thermal Gradient Management
Maintaining two distinct temperatures creates a thermal gradient between the zones.
If the transition between Zone I and Zone II is not managed well, phosphorus vapor may condense prematurely before reaching the precursor.
This requires careful positioning of the sample and accurate calibration of the furnace's thermal profile.
Optimizing Your Phosphorization Strategy
To effectively utilize a dual-zone CVD system for this application, consider your specific synthesis goals:
- If your primary focus is Phase Purity: Prioritize the stability of Zone II (400–700°C) to ensure the thermodynamic conditions favor the formation of Mn2P4O12.
- If your primary focus is Reaction Rate: Adjust the temperature of Zone I to modulate the sublimation rate of the phosphorus, thereby increasing or decreasing the vapor supply to the precursor.
Mastering the interplay between these two zones is the key to reproducible, high-quality material synthesis.
Summary Table:
| Feature | Zone I (Phosphorus Source) | Zone II (MnO2/CF Precursor) |
|---|---|---|
| Primary Function | Sublimation of P powder into vapor | Chemical transformation & synthesis |
| Temperature Goal | Control vapor supply rate | Facilitate reaction (400–700°C) |
| Key Outcome | Regulated phosphorization intensity | Phase-pure Mn2P4O12 microspheres |
| Critical Variable | Sublimation kinetics | Thermal stability & phase purity |
Elevate Your Material Synthesis with KINTEK
Precise thermal gradients are the difference between a failed experiment and a breakthrough in Mn2P4O12 cyclotetraphosphate synthesis. Backed by expert R&D and world-class manufacturing, KINTEK provides high-performance Dual-Zone Tube, Muffle, Rotary, and Vacuum CVD systems specifically engineered for complex phosphorization and chemical vapor deposition processes.
Our systems offer the independent temperature regulation and stability required to decouple sublimation from reaction kinetics, all customizable to meet your laboratory's unique research needs.
Ready to optimize your thin-film or powder synthesis?
→ Contact KINTEK Today for a Specialized Consultation
Visual Guide
References
- Kassa Belay Ibrahim, Alberto Vomiero. Electrochemically Modified Mn₂P₄O₁₂ as an Emerging Catalyst for Oxygen Evolution Reaction. DOI: 10.1002/admi.202500216
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Magnesium Extraction and Purification Condensing Tube Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- What are the material advantages of using a high-purity quartz tube reactor in continuous th-CVD systems?
- How are CVD processes categorized based on operational conditions? Explore Key Types and Applications
- What is chemical vapor deposition (CVD) and how does it work? Discover High-Performance Film Growth for Your Lab
- How is CVD used to coat glass? Achieve Durable, High-Volume Glass Coatings
- How does deposition occur on the substrate in CVD? Master Thin Film Growth with Precision Control
- What plasma methods are used in CVD processes? Discover Low-Temperature Solutions for Sensitive Substrates
- What industries commonly use CVD processes? Unlock High-Performance Thin Films for Your Sector
- What is the function of the External Heating Tape in 2D In2Se3 CVD? Master Precursor Control for Precision Synthesis



















