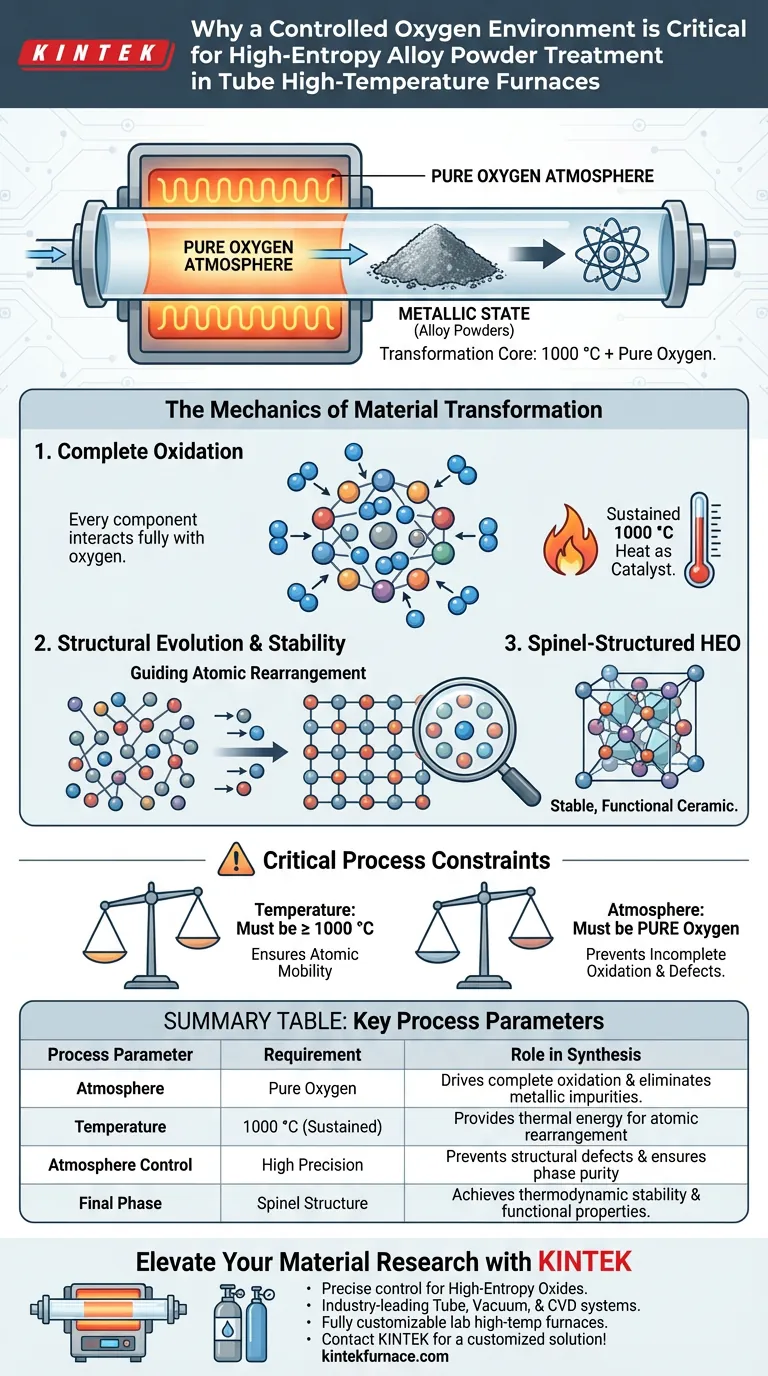
A controlled oxygen environment is the critical chemical driver required to transform high-entropy alloy powders into functional ceramics. Within a tube high-temperature furnace, this pure oxygen atmosphere, combined with sustained 1000 °C heat, facilitates the complete oxidation reaction necessary to convert the material from a metallic state into a stable high-entropy oxide (HEO).
The precise combination of high thermal energy and a pure oxygen atmosphere acts as a structural guide, forcing atomic rearrangement to create a stable, spinel-structured material.

The Mechanics of Material Transformation
Facilitating Complete Oxidation
The primary function of the controlled environment is to ensure a complete oxidation reaction.
High-entropy alloy powders are multi-component materials. To transition these from a metallic state to a functional oxide phase, every component must interact fully with oxygen. A pure oxygen atmosphere eliminates variables and ensures the reaction permeates the entire powder volume.
The Role of Thermal Energy
Heat acts as the catalyst for this transformation.
The tube furnace must maintain a sustained temperature of 1000 °C. This high thermal energy provides the necessary thermodynamics to drive the oxidation process efficiently and uniformly across the alloy powders.
Structural Evolution and Stability
Guiding Atomic Rearrangement
The synthesis of high-entropy oxides is not merely about burning metal; it is about architectural precision.
The specific conditions of the furnace guide atomic rearrangement. As the oxidation occurs, the atoms are coerced into moving from their metallic lattice positions into new, specific configurations.
Achieving the Spinel Structure
The ultimate goal of this treatment is the formation of a spinel-structured HEO.
This specific crystal structure is prized for its stability and functionality. The controlled oxygen and heat ensure the material settles into this stable phase rather than degrading into unstable byproducts or remaining partially metallic.
Critical Process Constraints
The Necessity of Precision
This process relies on the synergy between temperature and atmosphere.
If the temperature drops below 1000 °C, the atomic mobility may be insufficient to achieve the spinel structure. Conversely, if the oxygen atmosphere is impure, the oxidation may be incomplete, leading to structural defects or mixed phases that lack the desired functional properties.
Making the Right Choice for Your Synthesis
To ensure successful material processing, align your furnace parameters with your specific material goals:
- If your primary focus is Phase Purity: Ensure your oxygen supply is strictly controlled and pure to prevent incomplete oxidation or contamination.
- If your primary focus is Structural Stability: Verify that your furnace can sustain 1000 °C without fluctuation to guarantee the atomic rearrangement required for the spinel structure.
Success in creating high-entropy oxides lies in the rigorous control of the thermal and chemical environment.
Summary Table:
| Process Parameter | Requirement | Role in Synthesis |
|---|---|---|
| Atmosphere | Pure Oxygen | Drives complete oxidation and eliminates metallic impurities |
| Temperature | 1000 °C (Sustained) | Provides thermal energy for atomic rearrangement |
| Atmosphere Control | High Precision | Prevents structural defects and ensures phase purity |
| Final Phase | Spinel Structure | Achieves thermodynamic stability and functional properties |
Elevate Your Material Research with KINTEK
Precise atmospheric control and thermal stability are non-negotiable for the successful synthesis of high-entropy oxides. KINTEK provides industry-leading Tube, Vacuum, and CVD systems designed to maintain the rigorous 1000°C+ environments and pure gas flows your research demands.
Backed by expert R&D and manufacturing, our lab high-temp furnaces are fully customizable to meet your unique alloy processing needs. Ensure complete oxidation and structural stability in every batch.
Ready to optimize your synthesis? Contact KINTEK today for a customized solution!
Visual Guide
References
- Lenka Oroszová, Karel Saksl. Utilizing High-Capacity Spinel-Structured High-Entropy Oxide (CrMnFeCoCu)3O4 as a Graphite Alternative in Lithium-Ion Batteries. DOI: 10.3390/cryst14030218
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- What distinguishes an endothermic atmosphere from an exothermic atmosphere? Key Differences for Heat Treatment
- Why does a temperature of 800 degrees Celsius enable a near 100 percent conversion rate in the hydrogen reduction of tellurium dioxide?
- What is the role of a laboratory annealing furnace in memristor fabrication? Enhance Interface & Stability
- What are the risk mitigation strategies for atmosphere furnace operation? Ensure Safe and Efficient Lab Processes
- What process environment does a tube atmosphere furnace provide for LMFP? Master Secondary Crystallization
- How does the temperature control system work in the box type annealing atmosphere furnace? Achieve Precise Thermal Management
- What materials are used in the construction of a retort furnace? Discover Key Components for High-Temp Control
- What are the common heating methods used in atmosphere protection muffle furnaces? Choose the Right Method for Your Lab



















