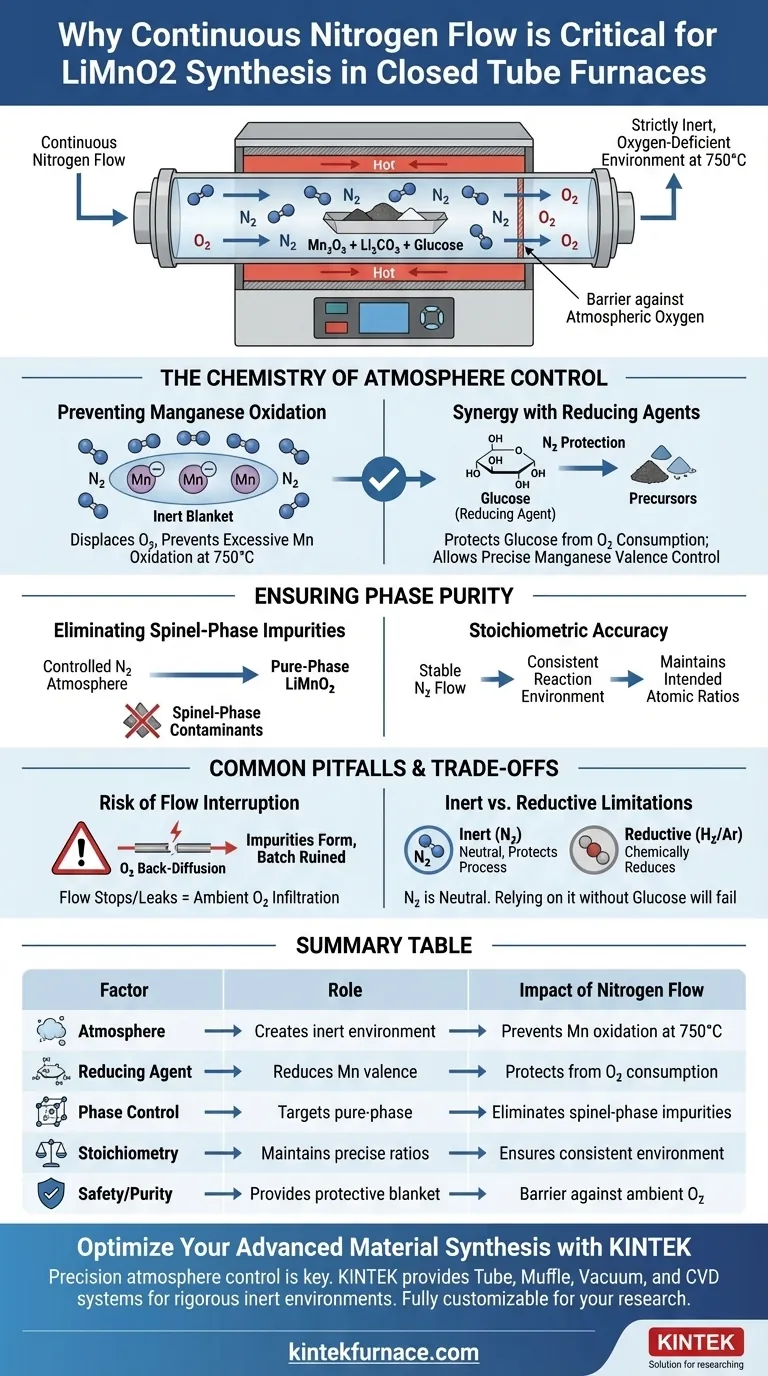
A continuous nitrogen flow is critical for creating a strictly inert, oxygen-deficient environment within the closed tube furnace. This protective atmosphere prevents the excessive oxidation of manganese ions during the 750°C synthesis stage, ensuring the successful formation of pure-phase LiMnO2.
The core function of the nitrogen flow is to act as a barrier against atmospheric oxygen. By maintaining an oxygen-deficient environment, you allow the reducing agent (glucose) to precisely control the manganese valence state, preventing the formation of unwanted impurities.

The Chemistry of Atmosphere Control
Preventing Manganese Oxidation
At high temperatures, specifically around 750°C, manganese ions are highly susceptible to excessive oxidation if exposed to air.
Continuous nitrogen flow displaces oxygen within the tube, creating an inert "blanket" around the reactants. This inhibition of oxidation is the fundamental prerequisite for synthesizing the correct material.
Synergy with Reducing Agents
Nitrogen alone provides the inert environment, but the chemical transformation relies on glucose acting as a reducing agent.
The nitrogen atmosphere ensures that the glucose reacts exclusively with the precursor materials (Mn2O3 and Li2CO3). It prevents the glucose from being consumed by atmospheric oxygen, preserving its reducing power for the synthesis of LiMnO2.
Ensuring Phase Purity
Eliminating Spinel-Phase Impurities
The primary risk in this synthesis is the formation of spinel-phase contaminants, which occur when manganese is allowed to over-oxidize.
By strictly controlling the atmosphere, the nitrogen flow forces the reaction path toward the desired pure-phase lithium manganite. This structural precision is vital for the electrochemical performance of the final material.
Stoichiometric Accuracy
For the reaction between Mn2O3 and Li2CO3 to yield the correct stoichiometry, external variables must be minimized.
A stable nitrogen flow ensures the reaction environment remains consistent throughout the heating process. This stability allows the precursors to react accurately, maintaining the intended atomic ratios in the final crystal lattice.
Common Pitfalls and Trade-offs
The Risk of Flow Interruption
The system relies on a continuous flow; a static nitrogen atmosphere is often insufficient.
If the flow stops or the tube is not perfectly sealed, ambient oxygen can diffuse back into the hot zone. Even trace amounts of oxygen at 750°C can trigger the formation of impurities, ruining the batch.
Inert vs. Reductive Limitations
It is important to distinguish between an inert atmosphere (Nitrogen) and a reductive atmosphere (like H2/Ar used for other precursors).
In this specific synthesis, nitrogen is neutral. It does not reduce the manganese itself; it merely protects the process so the added glucose can function effectively. Relying on nitrogen without the correct reducing agent would fail to produce LiMnO2.
Making the Right Choice for Your Goal
To ensure the success of your solid-phase synthesis, align your process controls with your purity requirements:
- If your primary focus is Phase Purity: Ensure the nitrogen flow is active before heating begins and continues until the furnace has completely cooled to prevent re-oxidation.
- If your primary focus is Stoichiometry: Verify that your glucose concentration is calculated correctly, as the nitrogen atmosphere relies on this agent to chemically reduce the manganese.
Control the atmosphere rigorously, and you control the quality of your final precursor.
Summary Table:
| Factor | Role in LiMnO2 Synthesis | Impact of Nitrogen Flow |
|---|---|---|
| Atmosphere | Creates inert/oxygen-deficient environment | Prevents excessive Mn oxidation at 750°C |
| Reducing Agent | Glucose reduces manganese valence | Protects glucose from atmospheric oxygen consumption |
| Phase Control | Targets pure-phase lithium manganite | Eliminates formation of spinel-phase impurities |
| Stoichiometry | Maintains precise atomic ratios | Ensures consistent reaction environment and stability |
| Safety/Purity | Provides protective gas blanket | Acts as a barrier against ambient oxygen diffusion |
Optimize Your Advanced Material Synthesis with KINTEK
Precision atmosphere control is the difference between pure-phase LiMnO2 and contaminated batches. KINTEK provides industry-leading Tube, Muffle, Vacuum, and CVD systems specifically designed to maintain the rigorous inert environments required for high-stakes research and production.
Backed by expert R&D and manufacturing, our high-temperature furnaces are fully customizable to meet your unique stoichiometric and thermal requirements. Don't let atmospheric contamination compromise your results.
Contact KINTEK Today to Customize Your Synthesis Solution
Visual Guide
References
- Jing Zhu, Run-Min Yao. Synthesis of Porous Lithium Ion Sieve with High Purity for Li+ Adsorption. DOI: 10.3390/ma18102373
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- What environmental conditions does a vacuum tube furnace provide for FTO(p)/ZnS(p) films? High-Purity Post-Treatment
- What role do tube furnaces play in the new energy and lithium materials industry? Essential for Precision Thermal Processing
- What is the role of a high-temperature Tube Furnace in copper alloy homogenization? Enhance Material Ductility
- Why do vacuum tube furnaces require strict pressure control for Borophene synthesis? Master Single-Phase Integrity
- How does the temperature control system in a tube furnace work? Master Precise Heating for Your Lab
- How does a tube furnace with programmable temperature control influence gas oil catalytic cracking? Optimize Your Yield
- Can you provide an example of a material prepared using a tube furnace? Discover YBa₂Cu₃O₇ Synthesis
- How does a laboratory high-temperature tube furnace contribute to the conversion of electrospun fibers? Expert Insights



















