The short answer is that Graphite Furnace Atomic Absorption Spectroscopy (GFAAS) is more sensitive than Flame AAS (FAAS) because it is far more efficient at converting a sample into measurable atoms and holds those atoms in the light path for a much longer time. This combination results in a stronger, more concentrated signal from a much smaller sample volume, allowing for the detection of analytes at parts-per-billion (ppb) levels or lower.
Graphite Furnace AAS achieves its superior sensitivity not through a single mechanism, but through two fundamental advantages over Flame AAS: near-perfect atomization efficiency and a significantly longer residence time for the atom cloud within the instrument's optical path.
The Core Principles of AAS Sensitivity
Atomic Absorption Spectroscopy works by measuring the light absorbed by free, ground-state atoms. Therefore, the sensitivity of any AAS technique is directly proportional to the number of free atoms generated from the sample and how long those atoms can be kept in the path of the light beam.
The more atoms in the beam, and the longer they stay there, the more light they will absorb, leading to a stronger signal and a lower detection limit.
How Flame AAS Limits Sensitivity
Flame AAS is a robust and rapid technique, but its design inherently limits its ultimate sensitivity. This limitation stems from two primary factors.
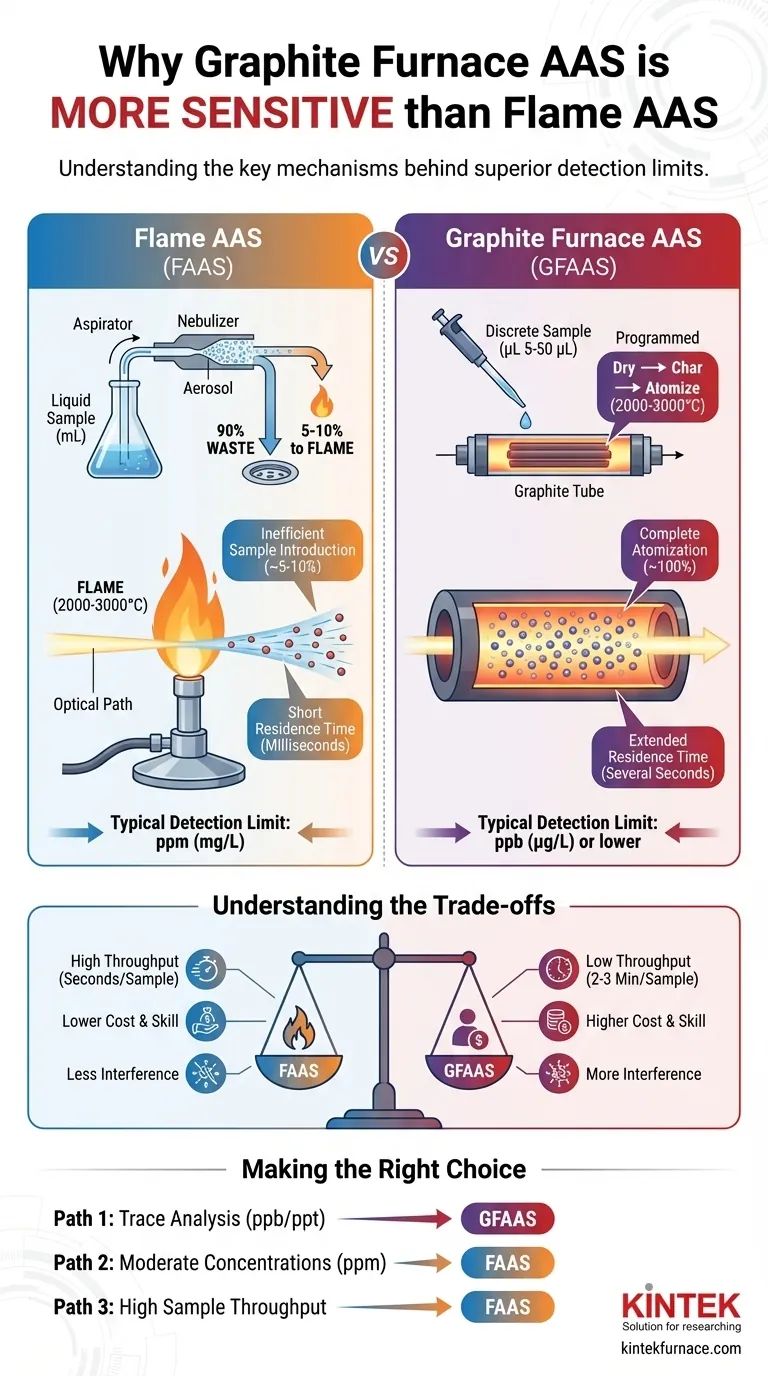
Inefficient Sample Introduction
In FAAS, the liquid sample is continuously aspirated into a nebulizer, which creates a fine aerosol. However, only about 5-10% of this aerosol is fine enough to be carried into the flame.
The vast majority of the sample, over 90%, condenses and goes to waste without ever being measured. This is a massive loss of potential signal before the analysis even begins.
Short Residence Time in the Flame
The atoms that are successfully created in the flame travel upwards with the hot gases at a very high velocity.
As a result, an individual atom remains in the instrument's light path for only a fraction of a second (milliseconds). The instrument is only measuring a brief, continuous "snapshot" of atoms as they rush through the observation zone.
How Graphite Furnace AAS Maximizes Sensitivity
GFAAS, also known as Electrothermal Atomization (ETA), was designed specifically to overcome the efficiency limitations of the flame method.
Complete Atomization of the Sample
Unlike the continuous aspiration in FAAS, GFAAS uses a discrete, small volume of sample (typically 5-50 microliters) injected directly into a graphite tube.
The tube is then heated in a programmed sequence to first dry the sample, then char away the matrix, and finally, to atomize virtually 100% of the analyte. No sample is wasted.
Extended Residence Time in the Tube
The graphite tube is a semi-enclosed environment. When the analyte is atomized at high temperature, it creates a dense cloud of atoms that is temporarily trapped within the confines of the tube.
This containment forces the atom cloud to remain in the instrument's light path for a much longer period—up to several seconds. This is hundreds of times longer than the residence time in a flame. This extended measurement window allows for a significantly greater total absorption signal to be recorded.
Understanding the Trade-offs: Sensitivity Isn't Everything
While GFAAS offers superior sensitivity, this performance comes with significant trade-offs. It is not always the better choice.
Speed and Sample Throughput
FAAS is exceptionally fast. Once calibrated, a sample can be analyzed in a matter of seconds. This makes it ideal for laboratories that need to process a high volume of samples quickly.
GFAAS is much slower. Each analysis requires a full temperature program cycle that can take 2 to 3 minutes per sample. This low throughput makes it unsuitable for rapid screening.
Susceptibility to Interference
The extended heating cycle and enclosed environment of the graphite furnace can lead to more complex chemical and spectral interferences from the sample matrix.
Developing a robust GFAAS method often requires more extensive optimization and the use of chemical modifiers to ensure accuracy. FAAS, with its high-temperature flame, is often more forgiving of complex sample matrices.
Cost and Operator Skill
Graphite furnace systems are more expensive to purchase and maintain than flame systems. The graphite tubes are consumable items with a limited lifetime and must be replaced regularly.
Operating a GFAAS system and developing methods also requires a higher level of operator skill and understanding of potential interferences.
Making the Right Choice for Your Analysis
Choosing between Flame AAS and Graphite Furnace AAS depends entirely on the analytical goal.
- If your primary focus is trace or ultra-trace analysis (ppb or ppt): GFAAS is the only viable choice due to its superior sensitivity and low sample volume requirements.
- If your primary focus is analyzing moderate-to-high concentrations (ppm): FAAS is the better choice, as its working range is perfectly suited for these levels and offers much higher speed.
- If your primary focus is high sample throughput: FAAS is the clear winner, capable of analyzing hundreds of samples in the time it would take to run a few dozen on a GFAAS system.
Ultimately, these two techniques are complementary tools, each designed to excel under different analytical conditions.
Summary Table:
| Feature | Flame AAS (FAAS) | Graphite Furnace AAS (GFAAS) |
|---|---|---|
| Atomization Efficiency | ~5-10% | ~100% |
| Atom Residence Time | Milliseconds | Several seconds |
| Typical Detection Limit | ppm (mg/L) | ppb (μg/L) or lower |
| Sample Volume | mL | μL (5-50 μL) |
| Sample Throughput | High (seconds/sample) | Low (2-3 minutes/sample) |
Need precise trace-element analysis? KINTEK's advanced laboratory furnaces, including high-temperature tube and vacuum models, provide the stable, controlled heating essential for developing reliable GFAAS methods. Our deep customization capabilities ensure your furnace meets exact thermal requirements for sensitive spectroscopic applications. Contact our experts today to discuss how our solutions can enhance your analytical precision.
Visual Guide
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- Split Multi Heating Zone Rotary Tube Furnace Rotating Tube Furnace
- Inclined Rotary Plasma Enhanced Chemical Deposition PECVD Tube Furnace Machine
People Also Ask
- What is the purpose of adding aluminum in the vacuum distillation process for magnesium? Enhancing Process Stability and Purity
- What is the significance of rapid quenching equipment in verifying the reaction pathway of BiFeO3? Capturing Intermediate Phases
- Why do Axial Flame Burners produce high NOx? Managing Thermal Intensity in Oxygen-Enhanced Combustion
- What is the function of a drying oven during the chemical activation of biochar? Optimize Your Porous Carbon Structure
- Why is a pressurized environment necessary for HMF synthesis? Ensure Liquid Phase Stability at High Temperatures
- What is the role of a rod mill during magnesite ore grinding? Achieve Optimal Flotation & Purification
- How does high-temperature filtration equipment facilitate molten salt separation? Boost Your Slag Treatment Recovery
- How does temperature control affect nanoporous copper dealloying? Master Pore Uniformity and Size



















