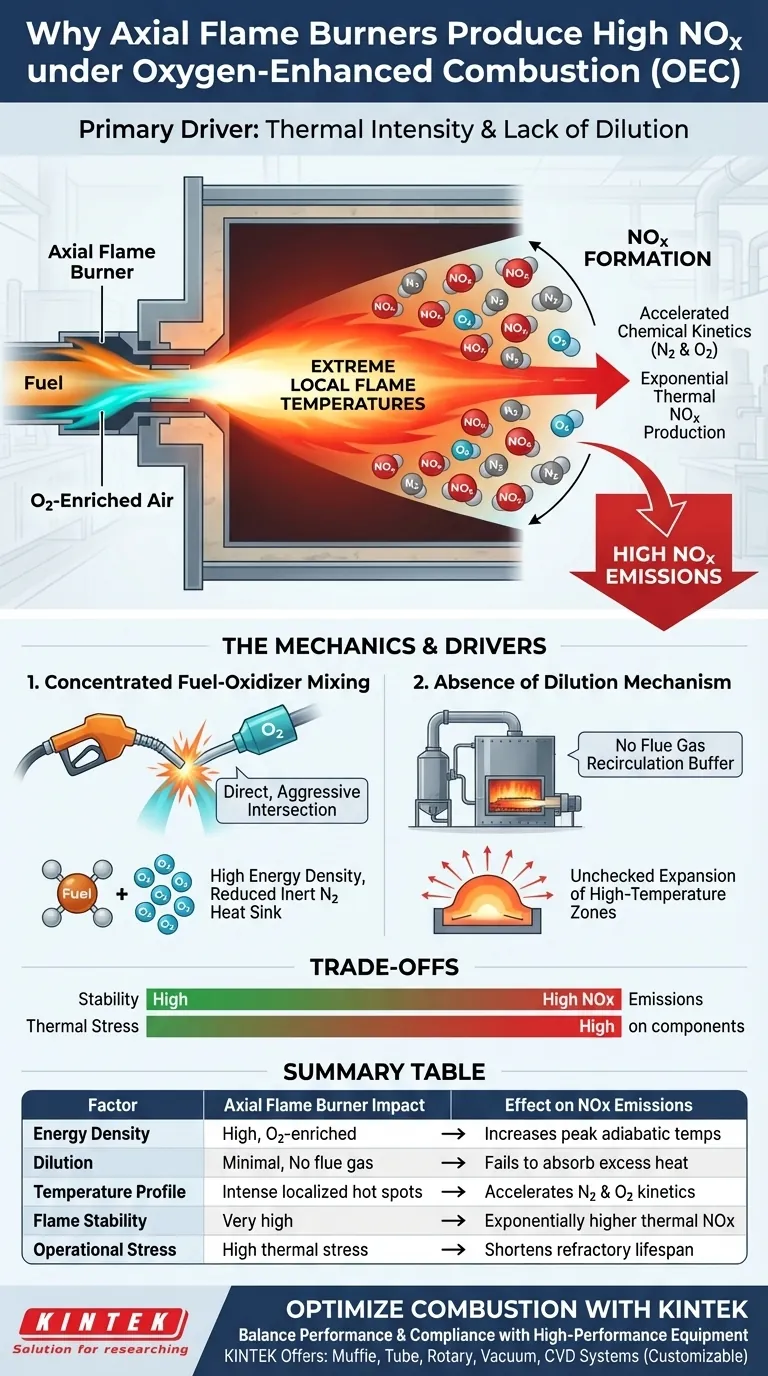
The primary driver is thermal intensity. Axial Flame Burners force a highly concentrated mixture of fuel and oxygen-enriched air to interact rapidly, creating extreme local flame temperatures. Because this configuration lacks a mechanism to dilute the flame with inert flue gases, these high-temperature zones expand unchecked, accelerating the chemical kinetics between nitrogen and oxygen to produce significant thermal NOx.
Under Oxygen-Enhanced Combustion (OEC), the absence of flue gas dilution in Axial Flame Burners results in intense, localized hot spots. This thermal environment acts as a catalyst, driving reaction kinetics that generate NOx levels far exceeding those found in milder combustion modes.

The Mechanics of High-Temperature Formation
Concentrated Fuel-Oxidizer Mixing
Axial Flame Burners are designed to create a direct and aggressive intersection of reactants.
Under OEC conditions, the fuel is mixed with oxygen-enriched air rather than standard air. This reduces the volume of inert nitrogen acting as a heat sink, leading to a much higher energy density within the flame.
The Absence of Dilution
A critical deficiency in this specific burner configuration is the lack of a flue gas dilution mechanism.
In lower-emission technologies, spent combustion gases are recirculated into the flame to lower its overall intensity. Axial burners do not employ this technique, meaning there is no buffer to absorb the heat generated during combustion.
How Heat Drives Emissions
Formation of High-Temperature Zones
Without dilution, the combustion process produces intense "hot spots" rather than a uniform temperature profile.
These zones represent peak adiabatic temperatures. Because the heat is not distributed or suppressed, these high-temperature areas expand significantly within the combustion chamber.
Accelerated Reaction Kinetics
The formation of NOx is thermally driven.
As the temperature within these expanded zones rises, the reaction kinetics between nitrogen and oxygen accelerate. This is not a linear relationship; the rate of thermal NOx production increases exponentially with temperature, making the undiluted heat of Axial Flame Burners particularly problematic for emissions control.
Understanding the Trade-offs
Stability vs. Emissions
While the intense mixing of Axial Flame Burners ensures a stable flame and robust combustion, it comes at an environmental cost. The very mechanism that ensures high combustion intensity—concentrated mixing—is directly responsible for the spike in NOx emissions.
Thermal Stress Implications
The same high-temperature zones that generate NOx also create operational challenges. The intense local heat can impose severe thermal stress on burner components and the surrounding refractory materials, potentially shortening equipment lifespan compared to MILD combustion modes.
Evaluating Burner Technology for Your Application
When selecting a combustion strategy, you must balance the need for heat intensity against regulatory emission limits.
- If your primary focus is high-intensity heat transfer: Recognize that the stability and heat density of Axial Flame Burners will likely require secondary gas treatment systems to manage the resulting NOx.
- If your primary focus is minimizing emissions: Investigate combustion modes that incorporate flue gas recirculation (such as MILD combustion) to suppress peak temperatures and inhibit thermal NOx formation.
Ultimately, controlling peak flame temperature through dilution is the most effective method for mitigating NOx in oxygen-enhanced environments.
Summary Table:
| Factor | Axial Flame Burner Impact | Effect on NOx Emissions |
|---|---|---|
| Energy Density | High (Oxygen-enriched fuel mixing) | Increases peak adiabatic temperatures |
| Dilution | Minimal (No flue gas recirculation) | Fails to buffer or absorb excess heat |
| Temperature Profile | Intense localized hot spots | Accelerates N2 and O2 reaction kinetics |
| Flame Stability | Very high and robust | Trade-off: Exponentially higher thermal NOx |
| Operational Stress | High thermal stress on components | Shortens lifespan of refractory materials |
Optimize Your Combustion Efficiency with KINTEK
Is your lab or production facility struggling with the trade-offs between heat intensity and NOx emissions? KINTEK provides the technical expertise and high-performance equipment you need to balance performance and compliance.
Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of lab high-temp furnaces, including Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet your unique research or industrial needs. Whether you require precise thermal control or low-emission combustion solutions, our engineers are ready to assist.
Contact KINTEK today to discover how our advanced furnace technology can enhance your operational efficiency!
Visual Guide
References
- Minsheng Zhao, Xianzhong Hu. Study on Flow and Heat Transfer Characteristics of Reheating Furnaces Under Oxygen-Enriched Conditions. DOI: 10.3390/pr13082454
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Small Rotary Kiln Calciner
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
People Also Ask
- What is the function of a forced air drying oven in the dehydration of acid-washed zeolite? Ensure Sample Purity.
- What are the advantages of combining vacuum hot rolling with small hole vacuuming? High-Bonding Clad Plate Production
- How does a vacuum pressure infiltration system contribute to Diamond/Cu composite green bodies? Achieve 60% Density
- Why must temperature loss be monitored during the aluminum alloy refining cycle? Essential Tips for Casting Success
- How does a molten salt bath furnace facilitate AISI 304 nitriding? Expert Guide to Superior Surface Hardness
- Why does the use of a forced-air drying oven often lead to increased particle size? Avoid Silica Agglomeration
- How does the temperature field provided by a High-Temperature Reaction Furnace promote pore development? 700-800°C Mastery
- Why is a laboratory vacuum drying oven required for perovskite nanopowders? Safeguard Nanostructure and Purity



















