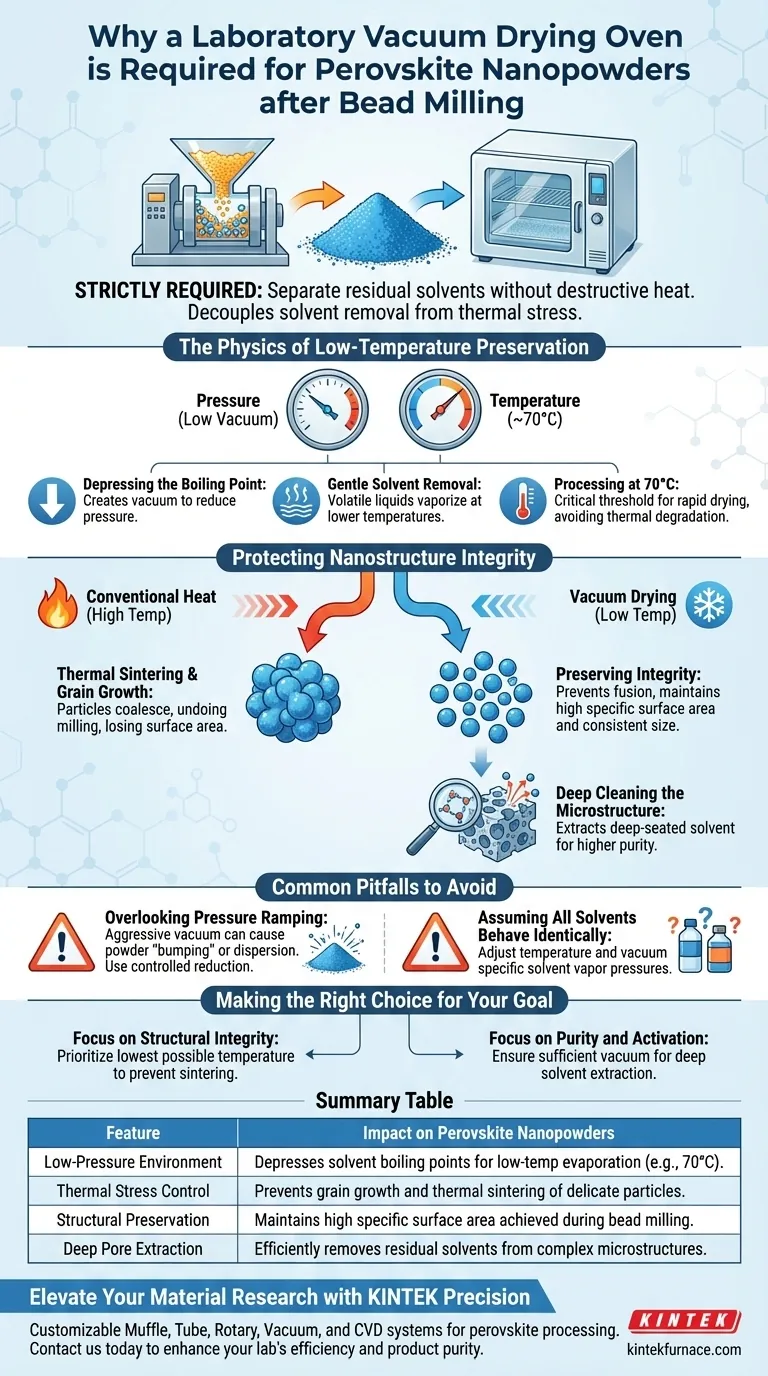
A laboratory vacuum drying oven is strictly required to separate residual solvents from bead-milled perovskite nanopowders without applying destructive heat. By operating under reduced pressure, you can rapidly evaporate solvents like ethanol at moderate temperatures (typically 70°C), safeguarding the delicate nanoscale features created during milling.
The core purpose of vacuum drying is to decouple solvent removal from thermal stress. It ensures the nanoparticles retain the specific surface area and structural integrity achieved during milling by preventing the grain growth associated with high-temperature drying.

The Physics of Low-Temperature Preservation
Depressing the Boiling Point
The fundamental advantage of this equipment is its ability to lower the boiling point of solvents. By creating a vacuum, the oven reduces the ambient pressure surrounding the wet nanopowders.
Gentle Solvent Removal
This pressure reduction allows volatile liquids, such as residual ethanol, to vaporize at significantly lower temperatures than they would at standard atmospheric pressure.
Processing at 70°C
In the specific case of perovskites, this allows for effective drying at temperatures around 70 degrees Celsius. This is a critical threshold that facilitates rapid drying without introducing the thermal energy that typically degrades nanomaterials.
Protecting Nanostructure Integrity
Preventing Thermal Sintering
High temperatures encourage nanoparticles to fuse together, a process known as thermal sintering. If perovskite powders are dried using conventional heat, the individual particles will likely coalesce, undoing the size reduction achieved by the bead milling process.
Mitigating Grain Growth
Vacuum drying inhibits grain growth, ensuring that the particle size remains consistent with your post-milling specifications. This is vital for maintaining the material's performance characteristics.
Preserving Specific Surface Area
The utility of nanopowders is often defined by their high specific surface area. By avoiding high heat and preventing particle fusion, the vacuum drying process preserves this critical metric.
Deep Cleaning the Microstructure
Beyond surface drying, the vacuum environment is effective at pulling solvent molecules out of deep pores or complex structures. This ensures a higher degree of material purity and activation.
Common Pitfalls to Avoid
Overlooking Pressure Ramping
While vacuum is essential, applying it too aggressively can cause fine nanopowders to disperse or "bump" inside the chamber. A controlled, gradual reduction in pressure is often necessary to keep the powder contained.
Assuming All Solvents Behave Identically
While ethanol responds well to this process at 70°C, other solvents may have different vapor pressure curves. You must adjust the temperature and vacuum levels based on the specific solvent used in your milling slurry.
Making the Right Choice for Your Goal
To maximize the quality of your perovskite nanopowders, align your drying parameters with your specific objectives:
- If your primary focus is Structural Integrity: Prioritize the lowest possible temperature that still achieves evaporation to strictly prevent sintering and grain growth.
- If your primary focus is Purity and Activation: Ensure the vacuum level is sufficient to extract deep-seated solvent molecules that might interfere with electrical property measurements.
By utilizing vacuum drying, you effectively lock in the benefits of the bead milling process, resulting in a pristine, high-performance nanomaterial.
Summary Table:
| Feature | Impact on Perovskite Nanopowders |
|---|---|
| Low-Pressure Environment | Depresses solvent boiling points for low-temp evaporation (e.g., 70°C). |
| Thermal Stress Control | Prevents grain growth and thermal sintering of delicate particles. |
| Structural Preservation | Maintains high specific surface area achieved during bead milling. |
| Deep Pore Extraction | Efficiently removes residual solvents from complex microstructures. |
Elevate Your Material Research with KINTEK Precision
Don't let high temperatures compromise the integrity of your engineered nanopowders. KINTEK provides industry-leading laboratory solutions tailored for advanced material science. Backed by expert R&D and manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet the rigorous demands of perovskite processing.
Ready to lock in the benefits of your milling process? Contact us today to discover how our specialized high-temp furnaces and vacuum ovens can enhance your lab's efficiency and product purity.
Visual Guide
References
- Sang‐Mun Jung, Yong‐Tae Kim. Low‐Temperature Exsolution of Cobalt From Perovskite Nanoparticles via Bead Milling for Enhanced Electrocatalytic Oxygen Evolution Reaction. DOI: 10.1002/adfm.202506227
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- 1200℃ Muffle Oven Furnace for Laboratory
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
People Also Ask
- What is the purpose of the rapid quenching process? Capture Precise High-Pressure Data Instantly
- What are the temperature ranges for low, medium, and high-temperature industrial heating processes? Optimize Your Process with KINTEK
- Why must (MnFeNiCo)3O4 materials undergo a secondary calcination? Key Steps to Optimizing FCC Spinel Structure
- What role does high-vacuum thermal evaporation equipment play in CsPbBr3 detectors? Optimize Electrode Fabrication
- What is the primary function of a laboratory electric drying oven in ACBP production? Ensure Precise Pre-treatment
- Why are User-Defined Functions (UDFs) necessary for modeling complex combustion? Unlock Precision in Furnace Simulation
- What is the necessity of baking electrode sheets in a vacuum oven? Ensure Battery Stability and Peak Performance
- What is the purpose of using a forced-air drying oven at 100 °C? Optimize Fe3O4@Fe-AC Composite Synthesis



















