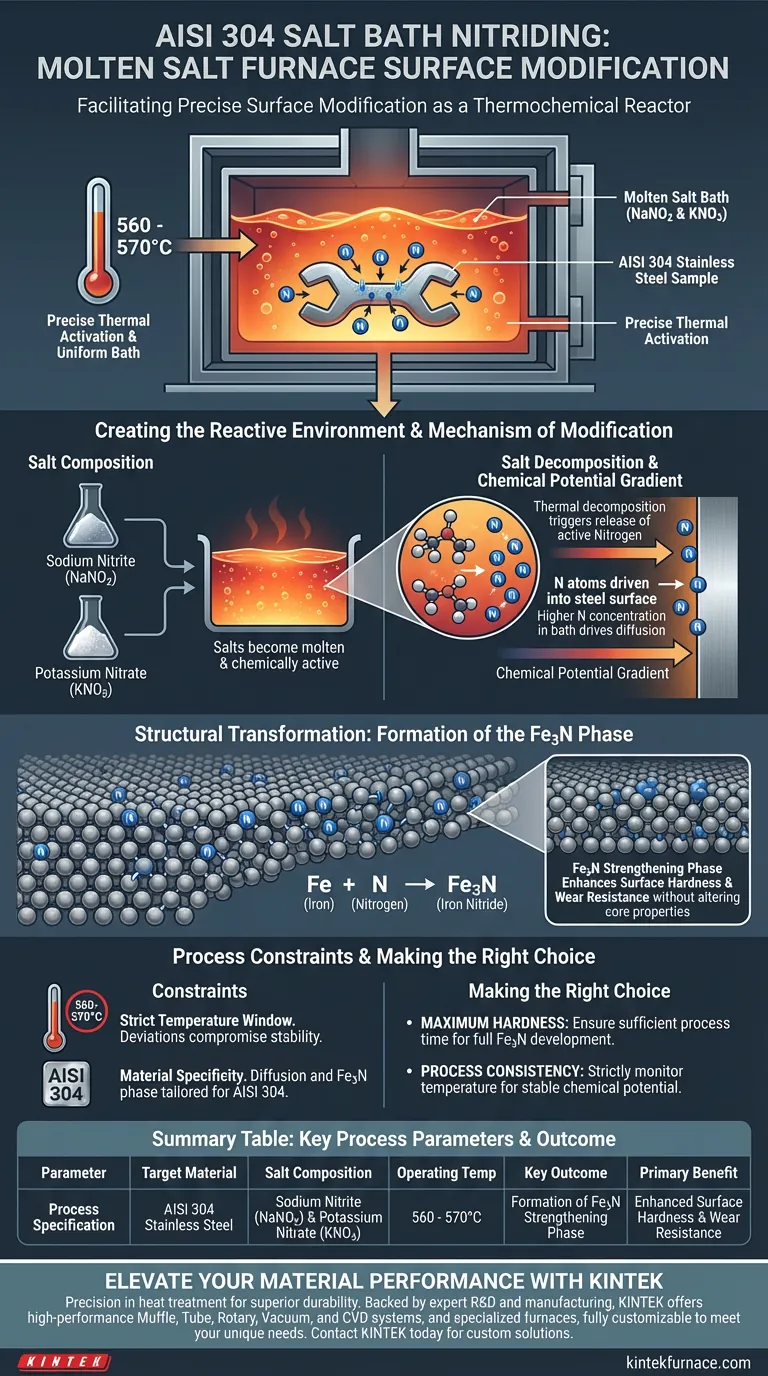
A molten salt bath furnace functions as a precise thermochemical reactor that facilitates surface modification by immersing AISI 304 stainless steel in a liquid mixture of Sodium Nitrite (NaNO2) and Potassium Nitrate (KNO3). By maintaining this mixture at a specific temperature range of 560-570°C, the furnace triggers the decomposition of nitrogen salts, allowing nitrogen atoms to diffuse directly into the steel's surface.
The core function of the furnace is to establish a high-temperature liquid environment where a chemical potential gradient drives nitrogen into the steel matrix. This results in the formation of an Fe3N strengthening phase, which significantly enhances the material's surface hardness.
Creating the Reactive Environment
The Role of Salt Composition
The process relies on a specific chemical mixture of Sodium Nitrite (NaNO2) and Potassium Nitrate (KNO3).
These salts are chosen because they become molten and chemically active within the target temperature range.
Precise Thermal Activation
The furnace heats this salt mixture to a strict temperature window of 560-570°C.
At this temperature, the salts transition from solid to liquid, creating a uniform bath that ensures even heat distribution across the surface of the submerged steel.
The Mechanism of Modification
Salt Decomposition
Once the bath reaches the operating temperature, the nitrogen-rich salts begin to decompose.
This thermal decomposition is the critical trigger that releases active nitrogen atoms from the compound, making them available for interaction with the steel.
Driven by Chemical Potential
The modification is driven by a chemical potential gradient.
Because the concentration of nitrogen is higher in the molten bath than in the steel, nitrogen atoms are naturally forced to diffuse into the surface of the AISI 304 samples.
Structural Transformation
Formation of the Fe3N Phase
As nitrogen diffuses into the steel lattice, it reacts chemically with the iron atoms.
This reaction forms Fe3N (Iron Nitride), a distinct microstructural phase known as the strengthening phase.
Enhancing Surface Hardness
The presence of the Fe3N phase is directly responsible for the change in mechanical properties.
This structural alteration significantly increases the surface hardness of the stainless steel, improving its resistance to wear without altering the core properties of the material.
Understanding Process Constraints
Temperature Sensitivity
The process relies heavily on maintaining the 560-570°C window.
Deviating from this range can compromise the stability of the liquid environment or fail to trigger the necessary salt decomposition.
Material Specificity
This specific mechanism is tailored for AISI 304 stainless steel.
The diffusion rates and formation of the Fe3N phase are specific to the interaction between this alloy's composition and the nitrate/nitrite salts.
Making the Right Choice for Your Goal
To maximize the benefits of salt bath nitriding for AISI 304 stainless steel, focus on these operational priorities:
- If your primary focus is Maximum Hardness: Ensure the process time is sufficient for the Fe3N strengthening phase to fully develop across the surface.
- If your primary focus is Process Consistency: strictly monitor the furnace temperature to stay within the 560-570°C range to maintain a stable chemical potential gradient.
By controlling the thermal and chemical environment precisely, you transform standard stainless steel into a highly wear-resistant material.
Summary Table:
| Parameter | Process Specification |
|---|---|
| Target Material | AISI 304 Stainless Steel |
| Salt Composition | Sodium Nitrite (NaNO2) & Potassium Nitrate (KNO3) |
| Operating Temp | 560 - 570°C |
| Key Outcome | Formation of Fe3N (Iron Nitride) Strengthening Phase |
| Primary Benefit | Enhanced Surface Hardness & Wear Resistance |
Elevate Your Material Performance with KINTEK
Precision in heat treatment is the difference between standard results and superior durability. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, along with specialized lab high-temp furnaces—all fully customizable to meet your unique AISI 304 surface modification needs.
Whether you require exact temperature control for salt bath nitriding or advanced vacuum solutions, our engineers provide the technology to drive your success. Contact KINTEK today to discuss your custom furnace solution.
Visual Guide
References
- G. Keerthi Reddy, Khristina Maksudovna Vafaeva. Influence of aisi 304 austenitic stainless steel by aqueous soluted nitriding and gas nitriding. DOI: 10.1051/matecconf/202439201019
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- Why is a two-stage sintering process used for porous LATP? Master Structural Integrity and Porosity
- Why is high-purity argon necessary for PVC dechlorination? Ensure Precise Reaction Control & Safety
- What is the function of a high-temperature heat treatment furnace? Optimize AlCuCrFe2NiTi0.25 Alloy Properties
- How does a precision temperature-controlled furnace regulate chemical composition in Cu-Cu2O heterostructures?
- What is the role of industrial thermometers in monitoring thermal stress? Ensure Safety via High-Precision Data
- What is the function of an inert gas supply system in black liquor pyrolysis? Achieve Precise Atmospheric Control
- What is the chemical vapor transport technique? A Guide to High-Purity Crystal Growth
- What is the primary role of a vacuum drying oven in WO3 nanostructure preparation? Achieve Perfect Morphology



















