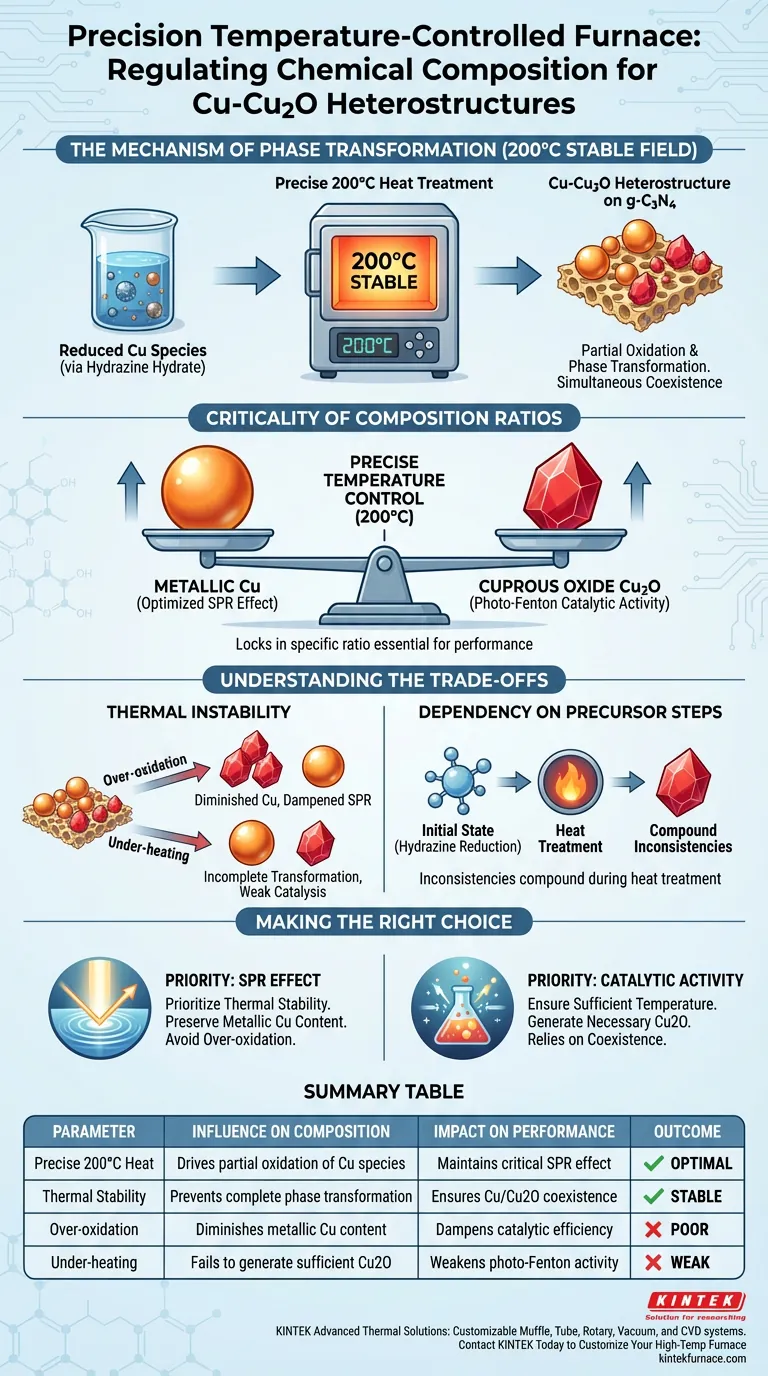
A precision temperature-controlled furnace regulates chemical composition by maintaining a strictly stable thermal field at 200°C. This specific thermal environment drives the partial oxidation and phase transformation of copper species that were previously reduced by hydrazine hydrate. By controlling the heat input, the furnace ensures the simultaneous coexistence of metallic copper (Cu) and cuprous oxide (Cu2O) on the graphitic carbon nitride (g-C3N4) surface.
The furnace acts as a phase-selector, preventing complete oxidation or reduction. It locks in a specific ratio of metallic Cu to Cu2O, which is essential for optimizing the material's surface plasmon resonance and catalytic performance.

The Mechanism of Phase Transformation
To understand the furnace's role, one must look at how heat dictates the chemical state of the copper.
Driving Partial Oxidation
The process begins with copper species generated through hydrazine hydrate reduction.
The furnace applies a consistent 200°C heat treatment to these species.
This temperature is calibrated to trigger a partial oxidation or phase transformation, rather than a total conversion.
Creating the Heterostructure
The result of this controlled heating is the formation of a heterostructure.
Metallic Cu and Cu2O are forced to coexist on the surface of the support material, graphitic carbon nitride (g-C3N4).
The furnace ensures that neither phase dominates completely, preserving the unique interface between the metal and the oxide.
The Criticality of Composition Ratios
The value of the furnace lies not just in heating, but in defining the exact ratio of chemical components.
Optimizing the Cu/Cu2O Ratio
Precise temperature control is the lever used to adjust the balance between reduced (Cu) and oxidized (Cu2O) states.
Any deviation in temperature would shift this equilibrium, altering the chemical composition of the final product.
Linking Composition to Performance
This specific chemical ratio is not arbitrary; it directly dictates the material's functional properties.
The coexistence of these two states is critical for maintaining the Surface Plasmon Resonance (SPR) effect.
Furthermore, this precise composition is required to enable the material's photo-Fenton catalytic activity.
Understanding the Trade-offs
While precision heating enables advanced material synthesis, it introduces specific sensitivities to the process.
The Risk of Thermal Instability
If the furnace fails to maintain the strict 200°C field, the chemical composition will drift.
Excessive heat could lead to over-oxidation, diminishing the metallic Cu content required for the SPR effect.
Insufficient heat may result in an incomplete phase transformation, failing to generate the necessary Cu2O for the heterostructure.
Dependency on Precursor Steps
The furnace's regulation is dependent on the initial state of the copper species.
Because the process relies on transforming species already reduced by hydrazine hydrate, inconsistencies in that reduction step can compound during the heat treatment.
Making the Right Choice for Your Goal
When configuring your thermal processing for Cu-Cu2O heterostructures, consider your specific performance targets.
- If your primary focus is Surface Plasmon Resonance (SPR): Prioritize thermal stability to preserve the metallic Cu content, as over-oxidation will dampen the resonance effect.
- If your primary focus is Photo-Fenton Catalytic Activity: Ensure the temperature is sufficient to generate the necessary Cu2O interface, as the catalytic mechanism relies on the coexistence of both oxidation states.
Precise thermal regulation is the defining factor that transforms a simple mixture of elements into a functional, high-performance heterostructure.
Summary Table:
| Parameter | Influence on Composition | Impact on Performance |
|---|---|---|
| Precise 200°C Heat | Drives partial oxidation of Cu species | Maintains critical SPR effect |
| Thermal Stability | Prevents complete phase transformation | Ensures Cu/Cu2O coexistence |
| Over-oxidation | Diminishes metallic Cu content | Dampens catalytic efficiency |
| Under-heating | Fails to generate sufficient Cu2O | Weakens photo-Fenton activity |
Unlock Material Precision with KINTEK Advanced Thermal Solutions
Precise chemical phase-selection demands a thermal environment that never wavers. KINTEK provides the industry-leading R&D and manufacturing expertise required to stabilize your most sensitive synthesis processes. Whether you are developing Cu-Cu2O heterostructures or advanced semiconductor materials, our customizable Muffle, Tube, Rotary, Vacuum, and CVD systems are designed to meet your exact specifications.
Ready to elevate your lab's catalytic and plasmonic research?
Contact KINTEK Today to Customize Your High-Temp Furnace
Visual Guide
References
- Guangying Zhou, Jianzhang Fang. Copper-Copper Oxide Heterostructural Nanocrystals Anchored on g-C3N4 Nanosheets for Efficient Visible-Light-Driven Photo-Fenton-like Catalysis. DOI: 10.3390/molecules30010144
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Vacuum Heat Treat Sintering and Brazing Furnace
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
People Also Ask
- What role does a laboratory drying oven play in the post-treatment of Cu/ZIF-8 catalysts? Ensuring Structural Integrity
- How does a needle valve control silver foil surface quality for graphene growth? Prevent defects with pressure control.
- Why use 10% Carbon Monoxide in black liquor pyrolysis? Prevent sodium volatilization for superior char quality.
- Why is MFI-type zeolite (S-1) selected for H-TiO2 synthesis? Master High-Efficiency Nanoparticle Templating
- What is the purpose of using a high-temperature universal material testing machine for Ti-6Al-4Zr-4Nb evaluation?
- What role do high-temperature sintering furnaces play in ceramic SLA? Unlock 99% Density in 3D Printed Ceramics
- What process problems are addressed by using a walking-beam furnace model? Solve Clad Plate Thermal Stress Challenges
- What is the temperature range of a lab furnace? Find Your Perfect Match



















