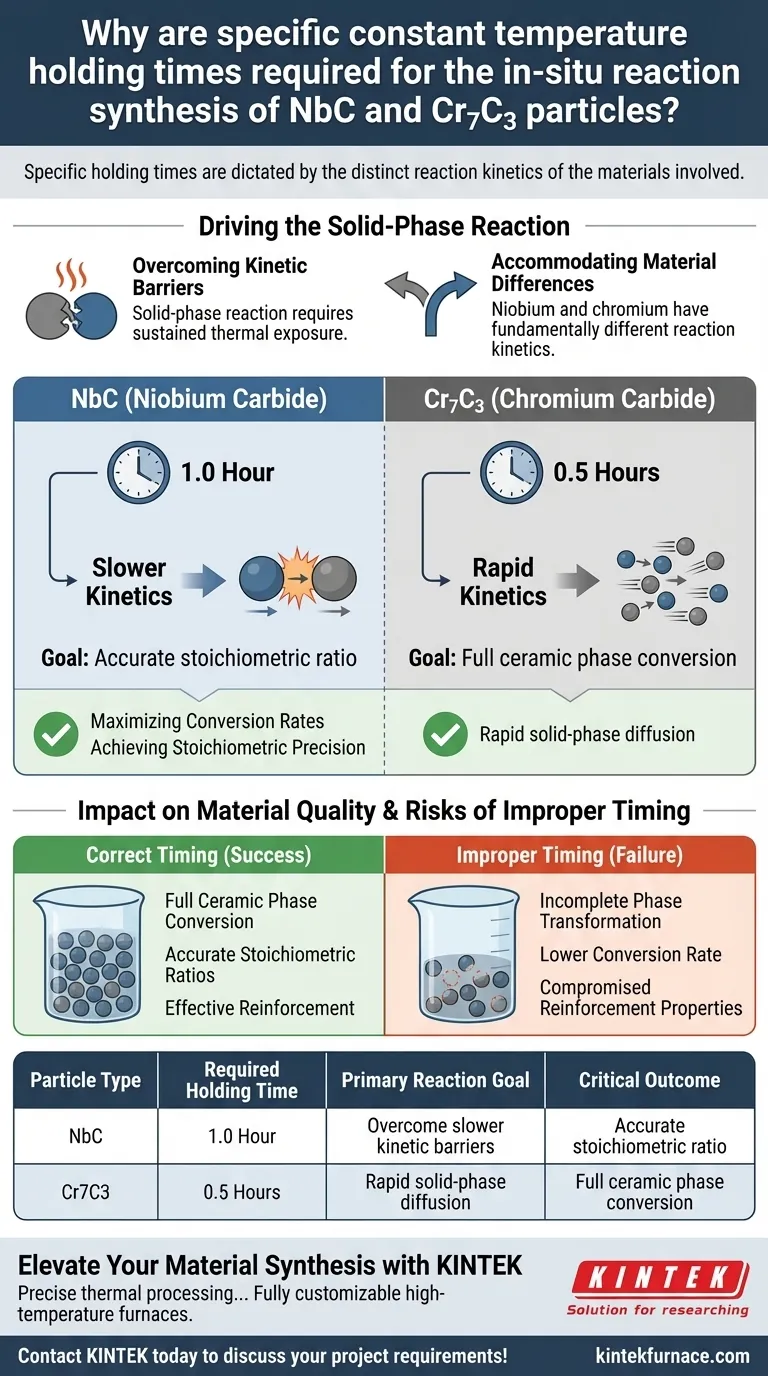
Specific holding times are dictated by the distinct reaction kinetics of the materials involved. For the in-situ synthesis of NbC and Cr7C3, precise durations—1 hour and 0.5 hours respectively—are required to ensure metal powders react completely with graphene in a solid-phase reaction. These specific windows allow for the full conversion of the ceramic phase based on how quickly each specific metal reacts with carbon.
The synthesis process relies on tailored holding times to accommodate the different reaction rates of niobium and chromium. Correct timing ensures a high conversion rate and accurate stoichiometric ratios, maximizing the strengthening potential of the ceramic particles.

Driving the Solid-Phase Reaction
Overcoming Kinetic Barriers
The synthesis process involves a solid-phase reaction between metal powders and graphene. Unlike liquid-phase reactions, these interactions require sustained thermal exposure to progress to completion.
Specific constant temperature holding times provide the necessary window for this diffusion and reaction to occur. Without adequate time at temperature, the physical interaction between the solid reactants cannot fully mature.
Accommodating Material Differences
Niobium and chromium possess fundamentally different reaction kinetics when interacting with carbon. Consequently, a "one-size-fits-all" approach is ineffective for generating high-quality ceramic particles.
Experimental data indicates that Cr7C3 requires a holding time of 0.5 hours to react fully. In contrast, the formation of NbC requires a longer duration of 1 hour to achieve the same level of completeness.
Impact on Material Quality
Maximizing Conversion Rates
The primary goal of the extended holding time, particularly for NbC, is to facilitate a higher conversion rate. The longer duration compensates for the slower kinetics of the niobium-carbon reaction.
By maintaining the temperature for the full hour, the process drives the transformation of raw metal and graphene into the desired ceramic phase.
Achieving Stoichiometric Precision
Accurate holding times are directly responsible for producing particles with accurate stoichiometric ratios. This chemical balance is critical for the stability and performance of the material.
When the reaction is allowed to run to completion, the resulting NbC ceramic particles possess the correct atomic composition, which is essential for their role as strengthening agents.
The Risks of Improper Timing
Incomplete Phase Transformation
If the holding time is curtailed, particularly for the slower-reacting niobium, the solid-phase reaction remains incomplete. This results in a lower conversion rate of the ceramic phase.
Compromised Reinforcement Properties
The ultimate purpose of these particles is to act as strengthening agents. Failing to adhere to the required holding times results in particles that lack the accurate stoichiometric ratios necessary to provide effective reinforcement.
Making the Right Choice for Your Goal
To ensure the successful synthesis of in-situ ceramic particles, you must adjust your process parameters based on the specific metal system you are utilizing.
- If your primary focus is synthesizing Cr7C3: A holding time of 0.5 hours is sufficient to ensure a full reaction between the chromium powder and graphene.
- If your primary focus is synthesizing NbC: You must extend the holding time to 1 hour to accommodate slower kinetics and ensure accurate stoichiometry.
Adhering to these material-specific time constraints is the only way to guarantee a fully converted, stoichiometrically accurate reinforcement phase.
Summary Table:
| Particle Type | Required Holding Time | Primary Reaction Goal | Critical Outcome |
|---|---|---|---|
| NbC (Niobium Carbide) | 1.0 Hour | Overcome slower kinetic barriers | Accurate stoichiometric ratio |
| Cr7C3 (Chromium Carbide) | 0.5 Hours | Rapid solid-phase diffusion | Full ceramic phase conversion |
Elevate Your Material Synthesis with KINTEK
Precise thermal processing is the difference between incomplete reactions and high-performance ceramic reinforcements. Whether you are synthesizing NbC, Cr7C3, or advanced composite materials, KINTEK provides the high-precision equipment necessary to maintain strict holding times and uniform temperatures.
Backed by expert R&D and world-class manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems. All our laboratory high-temperature furnaces are fully customizable to meet the unique kinetic requirements of your specific research.
Ready to optimize your ceramic synthesis? Contact KINTEK today to discuss your project requirements!
Visual Guide
References
- Lina Bai, Jie Liu. Effect of In Situ NbC-Cr7C3@graphene/Fe Nanocomposite Inoculant Modification and Refinement on the Microstructure and Properties of W18Cr4V High-Speed Steel. DOI: 10.3390/ma17050976
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- Why is a heating system with closed-loop feedback essential for TL analysis? Precision Tips for High-Accuracy Kinetics
- What is the function of a magnetron sputtering system for WS2 thin films? Master Nano-Scale Deposition Control
- What is the purpose of using a vacuum dryer for PU and AlN composite sheets? Enhance Thermal & Structural Integrity
- What is the purpose of performing a final annealing treatment? Optimize Your Heterojunction Device Performance
- What are the energy consumption considerations when choosing between separate or combined debinding and sintering furnaces? Optimize Your Process Efficiency
- Why are DEZ and GEME selected for Ge:ZnO ALD? Unlock Precise Atomic Doping and Thermal Stability
- Why is substrate preheating typically employed during the LPBF process? Minimize Stress & Prevent Cracks in 3D Printing
- What is the primary function of a constant temperature drying oven in ceramic powder pretreatment? Get Expert Results



















