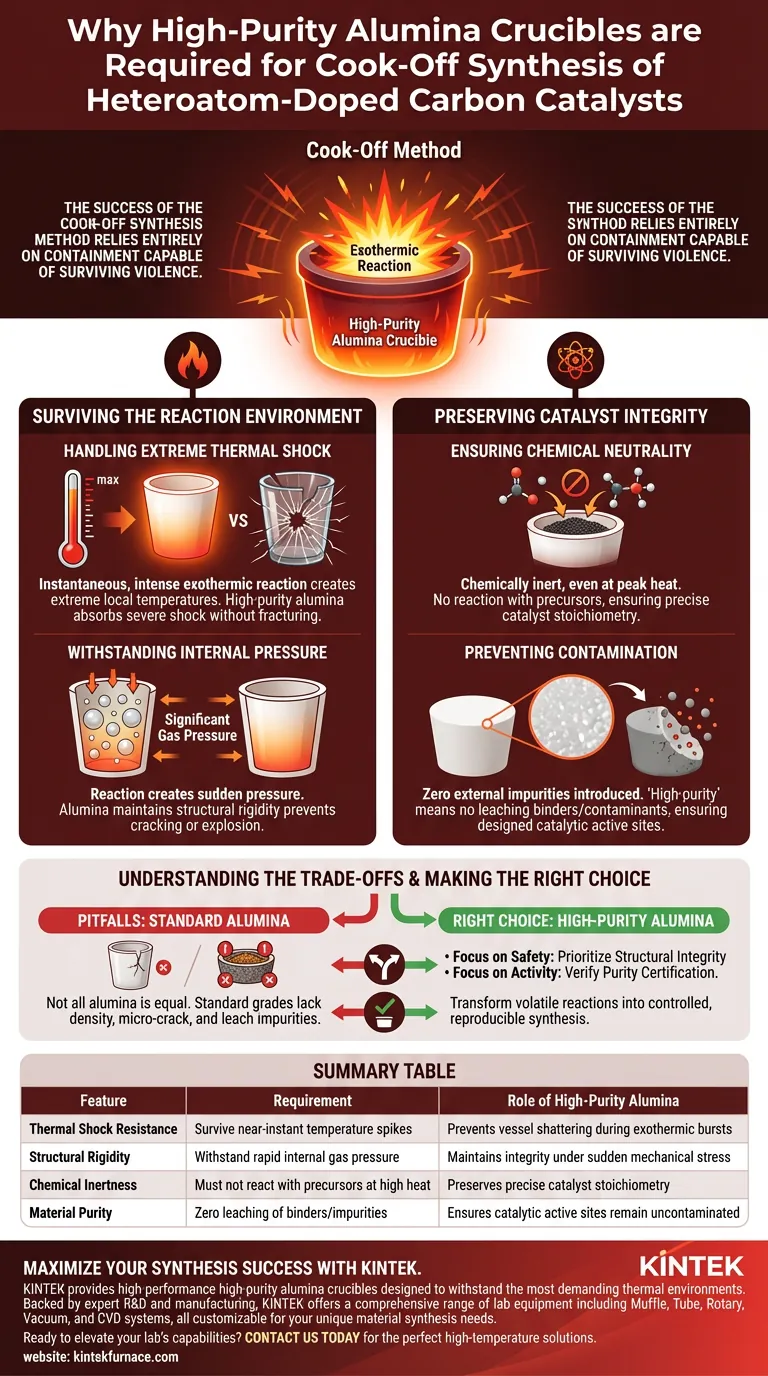
The success of the cook-off synthesis method relies entirely on containment capable of surviving violence. High-purity alumina crucibles are strictly required because they possess the unique ability to withstand the extreme thermal shock generated by the instantaneous, intense exothermic reactions characteristic of this method. Their use prevents the vessel from shattering under rapid temperature spikes and gas pressure while ensuring the catalyst remains chemically pure.
The cook-off method generates immediate, localized heat and pressure that would destroy standard labware. High-purity alumina is essential not just for high-temperature resistance, but specifically for its ability to survive this rapid energy release without introducing contaminants into your catalyst.

Surviving the Reaction Environment
Handling Extreme Thermal Shock
The cook-off method is distinguishable by its speed; it involves an intense exothermic reaction that occurs almost instantly.
This generates extremely high local temperatures in a fraction of a second. Standard glass or lower-grade ceramics would likely fracture due to the rapid differential expansion. High-purity alumina is structurally capable of absorbing this severe thermal shock without mechanical failure.
Withstanding Internal Pressure
The reaction does not just generate heat; it creates significant gas pressure instantaneously.
The crucible must act as a pressure vessel during the synthesis spike. Alumina maintains its structural rigidity even when subjected to the dual stress of sudden heating and expanding internal gases, preventing the container from cracking or exploding.
Preserving Catalyst Integrity
Ensuring Chemical Neutrality
In the synthesis of heteroatom-doped carbon catalysts, the chemical composition must be precise.
High-purity alumina is chemically inert. Even at the peak temperatures of the cook-off reaction, it refuses to react with the precursors. This ensures that the stoichiometry of your catalyst is dictated solely by your reactants, not by interaction with the container wall.
Preventing Contamination
The "high-purity" aspect of the crucible is as critical as the material itself.
Lower-quality crucibles often contain binders or impurities that can leach out under stress. High-purity alumina ensures that no external impurities are introduced during synthesis. This guarantees that the catalytic active sites are formed exactly as designed, without interference from foreign elements.
Understanding the Trade-offs
While high-purity alumina is the superior choice for this method, it is important to recognize potential pitfalls regarding material grades.
Not all alumina is created equal. A common mistake is substituting standard lab-grade alumina for high-purity variants to reduce costs. Standard alumina often lacks the density to withstand the specific violence of the cook-off method, leading to micro-cracking. Furthermore, trace impurities in lower-grade vessels can alter the electronic structure of your doped carbon, rendering the catalyst less effective.
Making the Right Choice for Your Goal
To ensure reproducible results in your synthesis, align your equipment choice with your specific experimental needs:
- If your primary focus is safety and containment: Prioritize the structural integrity of the crucible to ensure it can survive the rapid expansion of gases and heat without catastrophic failure.
- If your primary focus is catalyst activity: Verify the purity certification of the alumina to ensure zero leaching of contaminants that could poison the active sites of your doped carbon.
By selecting the correct vessel, you transform a volatile reaction into a controlled, reproducible synthesis technique.
Summary Table:
| Feature | Requirement for Cook-off Method | Role of High-Purity Alumina |
|---|---|---|
| Thermal Shock Resistance | Must survive near-instant temperature spikes | Prevents vessel shattering during exothermic bursts |
| Structural Rigidity | Must withstand rapid internal gas pressure | Maintains integrity under sudden mechanical stress |
| Chemical Inertness | Must not react with precursors at high heat | Preserves precise catalyst stoichiometry |
| Material Purity | Zero leaching of binders or metallic impurities | Ensures catalytic active sites remain uncontaminated |
Maximize Your Synthesis Success with KINTEK
Don't let inferior labware compromise your research safety or catalyst performance. KINTEK provides high-performance high-purity alumina crucibles designed to withstand the most demanding thermal environments.
Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of lab equipment including Muffle, Tube, Rotary, Vacuum, and CVD systems, all customizable for your unique material synthesis needs.
Ready to elevate your lab's capabilities? Contact us today to find the perfect high-temperature solutions for your next breakthrough.
Visual Guide
References
- Ruiquan Zhang, Maocong Hu. Heteroatom-Doped Carbon-Based Catalysts Synthesized through a “Cook-Off” Process for Oxygen Reduction Reaction. DOI: 10.3390/pr12020264
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Magnesium Extraction and Purification Condensing Tube Furnace
- Chairside Dental Porcelain Zirconia Sintering Furnace with Transformer for Ceramic Restorations
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- How does a laboratory high-temperature oven facilitate the destabilization of lignin? Optimize Biomass Pretreatment
- Why is a high-precision mass flow controller (MFC) necessary in ferronickel alloy smelting? Ensure Metal Purity
- What cost factors should be considered when choosing an alumina ceramic furnace tube? Optimize Total Cost of Ownership
- Why is a laboratory pellet press used to compress powders? Optimize Conductivity for Flash Joule Heating
- How do stainless steel furnace chambers and insulating linings contribute to thermal design? Boost Energy Efficiency
- Why are fly ash geopolymer specimens subjected to 60 °C drying? Master Accelerated Curing for Maximum Strength
- What role does a laboratory hydraulic press play in manufacturing nickel composites? Achieving Maximum Density
- Why are vacuum filtration devices and specific cellulose filter papers used in hydrothermal synthesis recovery?
















